Ontogeny of Photophore Pattern in the Velvet Belly Lantern Shark, Etmopterus Spinax Julien M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught. -

Coelho Phd Lantern S
UNIVERSIDADEdo ALGARVE FaculdadedeCiênciasdoMaredo Ambiente Biology,populationdynamics,managementandconservation ofdeepwaterlanternsharks,Etmopterusspinax and Etmopteruspusillus (Chondrichthyes:Etmopteridae)insouthernPortugal(northeastAtlantic). (DoutoramentoemCiênciaseTecnologiasdasPescas,especialidadedeBiologiaPesqueira) (ThesisforthedegreeinDoctorofPhilosophyinFisheriesSciencesandTechnologies,specialtyinFisheriesBiology) RUIPEDROANDRADECOELHO Faro (2007) UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS DO MAR E DO AMBIENTE Biology, population dynamics, management and conservation of deep water lantern sharks, Etmopterus spinax and Etmopterus pusillus (Chondrichthyes: Etmopteridae) in southern Portugal (northeast Atlantic). (Doutoramento em Ciências e Tecnologias das Pescas, especialidade de Biologia Pesqueira) (Thesis for the degree in Doctor of Philosophy in Fisheries Sciences and Technologies, specialty in Fisheries Biology) RUI PEDRO ANDRADE COELHO Orientador / Supervisor: Prof. Doutor Karim Erzini Júri / Jury: - Prof. Doutor José Pedro Andrade, Professor Catedrático da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Karim Erzini, Professor Associado com Agregação da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Leonel Paulo Sul de Serrano Gordo, Professor Auxiliar com Agregação da Faculdade de Ciências, Universidade de Lisboa; - Prof. Doutor Manuel Seixas Afonso Dias, Professor Auxiliar da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; -
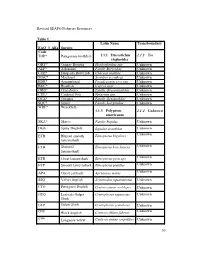
Revised SEAFO Fisheries Resources Table 1. . FAO 3 Alfa Species Code
Revised SEAFO Fisheries Resources Table 1. Latin Name Transboundary FAO 3 Alfa Species Code TOP* Patagonian toothfish 1.1.1 Dissostichus 1.1.2 Yes eleginoides ORY* Orange Roughy Hoplosthethus spp Unknown ALF* Alfonsino Family Berycidae Unknown CGE* Deep-sea Red Crab Chaceon maritae Unknown MAC* Mackerel Scomber scombrus Unknown EDR* Armourhead Pseudopentaceros spp. Unknown BOC* Boarfish Capros aper Unknown ORD* Oreo dories Family Oreosomatidae Unknown CDL* Cardinal Fish Epigonus spp. Unknown OCZ* Octopus Family Octopodidae Unknown SQC* Squid Family Loliginidae Unknown WRF* Wreckfish 1.1.3 Polyprion 1.1.4 Unknown americanus SKA* Skates Family Rajidae Unknown DGS Spiny Dogfish Squalus acanthias Unknown Unknown ETB Blurred smooth Etmopterus bigelowi lanternshark ETH Shorttail Etmopterus brachyurus Unknown lanternshark ETR Great lanternshark Etmopterus princeps Unknown ETP Smooth lanternshark Etmopterus pusillus Unknown Unknown APA Ghost catshark Apristurus manis SSQ Velvet dogfish Scymnodon squamulosus Unknown CYO Portuguese Dogfish Centroscymnus coelolepis Unknown GUQ Leafscale Gulper Centrophorus squamosus Unknown Shark GUP Gulper Shark Centrophorus granulosus Unknown CFB ǂ Black dogfish Centroscyllium fabricii Unknown CYP ǂ Longnose velvet Centroscymnus crepidater Unknown 30 dogfish CYY Unknown ǂ Shortnose velvet Centroscymnus dogfish cryptacanthus SCK ǂ Kitefin shark Dalatias licha Unknown ETE ǂ Etmopterus compagnoi Unknown ETI ǂ Broadbanded Etmopterus gracilispinis Unknown lanternshark ETM ǂ Unknown Southern Etmopterus granulosus lanternshark -

251 Part 640—Spiny Lobster Fishery of the Gulf Of
Fishery Conservation and Management Pt. 640 of an application for an ILAP or an ap- Sevengill, Heptranchias perlo peal of NMFS's denial of an initial lim- Sixgill, Hexanchus griseus ited access permit for swordfish. Smalltail, Carcharhinus porosus (12) Falsify information submitted Whale, Rhincodon typus White, Carcharodon carcharias under § 635.46(b) in support of entry of imported swordfish. TABLE 2 OF APPENDIX A TO PART 635± (13) Exceed the incidental catch re- DEEPWATER/OTHER SHARK SPECIES tention limits specified at § 635.24(b). Blotched catshark, Scyliorhinus meadi [64 FR 29135, May 28, 1999, as amended at 64 Broadgill catshark, Apristurus riveri FR 37705, July 13, 1999; 65 FR 42887, July 12, Chain dogfish, Scyliorhinus retifer Deepwater catshark, Apristurus profundorum 2000; 65 FR 47238, Aug. 1, 2000] Dwarf catshark, Scyliorhinus torrei Iceland catshark, Apristurus laurussoni APPENDIX A TO PART 635ÐSPECIES Marbled catshark, Galeus arae TABLES Smallfin catshark, Apristurus parvipinnis TABLE 1 OF APPENDIX A TO PART 635±OCEANIC Bigtooth cookiecutter, Isistius plutodus Blainville's dogfish, Squalus blainvillei SHARKS Bramble shark, Echinorhinus brucus A. Large coastal sharks: Broadband dogfish, Etmopterus gracilispinnis Caribbean lanternshark, Etmopterus hillianus 1. Ridgeback sharks: Cookiecutter shark, Isistius brasiliensis Sandbar, Carcharhinus plumbeus Cuban dogfish, Squalus cubensis Silky, Carcharhinus falciformis Flatnose gulper shark, Deania profundorum Tiger, Galeocerdo cuvieri Fringefin lanternshark, Etmopterus schultzi Great -

And Their Functional, Ecological, and Evolutionary Implications
DePaul University Via Sapientiae College of Science and Health Theses and Dissertations College of Science and Health Spring 6-14-2019 Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications Phillip C. Sternes DePaul University, [email protected] Follow this and additional works at: https://via.library.depaul.edu/csh_etd Part of the Biology Commons Recommended Citation Sternes, Phillip C., "Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications" (2019). College of Science and Health Theses and Dissertations. 327. https://via.library.depaul.edu/csh_etd/327 This Thesis is brought to you for free and open access by the College of Science and Health at Via Sapientiae. It has been accepted for inclusion in College of Science and Health Theses and Dissertations by an authorized administrator of Via Sapientiae. For more information, please contact [email protected]. Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Master of Science June 2019 By Phillip C. Sternes Department of Biological Sciences College of Science and Health DePaul University Chicago, Illinois Table of Contents Table of Contents.............................................................................................................................ii List of Tables..................................................................................................................................iv -

© Iccat, 2007
A2.2 ICCAT Species Codes APPENDIX 2.2: SPECIES CODES Y ello wfin tuna Codes used to identify the ICCAT tuna and tuna-like species as well as by-catch species Atún blanco Tuna and tuna-like species G e r m o n Numerical Alphabetical Scientific Name English SkipjackFra tunancais EspañolR a b i l 1 BFT Thunnus thynnus Northern bluefin tuna Thon rouge du Nord Atún común (Cimarrón) 2 SBF Thunnus maccoyii Southern bluefin tuna Thon rouge du Sud Atún del Sur 3 YFT Thunnus albacares erocablA T hazard-bâtard L i s t a d o 4 ALB Thunnus alalunga erocablA Plain bonito 5 BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo)P a l o m e t tPatudo e 6 BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras 7 LTA Euthynnus alletteratus Little tunny(=Atl.black skipjack) Thonine commune BacoretaT a s a r t e 8 SKJ Katsuwonus pelamis WBlack a h o o m arlinoatsiL M akaire noir 9 BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del AtlánticoA guja negra P e t o 10 FRI Auxis thazard Frigate tuna Auxide Melva 11 BOP Orcynopsis unicolor 12 WAH Acanthocybium solandri Pez espada 13 SSM Scomberomorus maculatus Atlantic SpanishS w mackerel o r d f i s hTh azard atlantique Carite atlántico 14 KGM Scomberomorus cavalla King mackerel Thazard Ebarr sé p a d o n Carite lucio 15 SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico 16 BLM Makaira indica 17 BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico 18 WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico 28 19 SWO Xiphias gladius 3 20 SPF Tetrapturus pfluegeri Longbill spearfish Makaire bécune Aguja picuda 284 ICCAT MANUAL, 1st Edition (January 2010) 21 TUN Thunnini sanuT ien sédinohT acn senutA pen 23 YOU gnuoY sanut senueJ sédinoht senutA senevój 24 BIL Istiophoridae Marlins,sailfishes,etc. -

Identification Guide to the Deep-Sea Cartilaginous Fishes of the Indian
Identification Guide to the Deep–Sea Cartilaginous Fishes of the Indian Ocean Ebert, D.A. and Mostarda, E. 2013. Identification guide to the deep–sea cartilaginous fishes of the Indian Ocean. FishFinder Programme, FAO, Rome. 76 pp. Supervision: Merete Tandstad, Jessica Sanders and Johanne Fischer (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme”, thanks to a generous funding from the Governments of Norway and Japan (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas and Fisheries management and marine conservation within a changing ecosystem context projects) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). Its production is the result of a collaborative effort among scientists, fishery observers and the fishing industry who attended the FAO regional workshop held in Flic en Flac, Mauritius, from January 16 to 18, 2013. The general objective of the workshop was to discuss, share experiences and finally draft recommendations for the development of field products aimed at facilitating the identification of Indian Ocean deep-sea cartilaginous fishes. The present guide covers the deep–sea Indian Ocean, primarily FAO Fishing Areas 51 and 57, and that part of Area 47 that extends from Cape Point, South Africa to the east, e.g. -

Sharks1 (Sharks)
CMM 2019/12 Conservation and Management Measure for Sharks1 (Sharks) The Meeting of the Parties to the Southern Indian Ocean Fisheries Agreement: RECALLING the relevant provisions of the Southern Indian Ocean Fisheries Agreement, in particular Article 4; CONSIDERING that the United Nations Food and Agriculture Organization (FAO) International Plan of Action for Sharks calls on States to cooperate through regional fisheries organizations to ensure the sustainability of shark stocks; RECOGNIZING the need to improve the collection of species-specific data on catch, effort, discards, and trade as a basis for improving the conservation and management of shark stocks; RECALLING that the FAO International Plan of Action for Sharks calls on States to encourage full use of dead sharks, to facilitate improved species-specific catch and landings data and monitoring of shark catches and the identification and reporting of species-specific biological and trade data; FURTHER RECALLING that United Nations General Assembly, adopted consensus Resolutions every year since 2007 (62/177, 63/112 , 64/72, 65/38, 66/68, 67/79, 68/71, 69/109, 70/75 and 71/123), calling upon States to take immediate and concerted action to improve the implementation of and compliance with existing regional fisheries management organization or arrangement measures that regulate shark fisheries and incidental catch of sharks, in particular those measures which prohibit or restrict fisheries conducted solely for the purpose of harvesting shark fins, and, where necessary, to consider taking other measures, as appropriate, such as requiring that all sharks be landed with each fin naturally attached; ADOPTS the following Conservation and Management Measures (CMM) in accordance with Article 4 and 6 of the Agreement: 1. -

APPENDIX D – Provisional SEAFO Species List
APPENDIX D – Provisional SEAFO Species List FAO 3 Alfa Groups Common Name Family Species Author Code SSH Scarlet shrimp Aristeidae Aristaeopsis edwardsiana (J. Y. Johnson, 1868) ARI ‐ Aristeidae Austropenaeus nitidus (Barnard, 1947) (Geryons ‐Red/Golden deep‐sea (GER) Geryonidae Chaceon chuni (Macpherson, 1983) crabs) (Geryons ‐Red/Golden deep‐sea (GER) Geryonidae Chaceon erytheiae (Macpherson, 1984) crabs) (Geryons ‐Red/Golden deep‐sea (GER) Geryonidae Chaceon gordonae (Ingle, 1985) crabs) (Geryons ‐Red/Golden deep‐sea (GER) Geryonidae Chaceon sanctaehelenae Manning and Holthuis, 1989 crabs) Crustaceans OLV Paromola Homolidae Paromola cuvieri Wood‐Mason and Alcock, 1891 KCA King crab Lithodidae Lithodes ferox (Filhol, 1885) (A. Milne‐Edwards and Bouvier, NLE Rough king crab Lithodidae Neolithodes asperrimus 1894) KCX (King crabs) Lithodidae Neolithodes capensis Benedict, 1895 KCM Subantarctic stone crab Lithodidae Lithodes murrayi Henderson, 1888 KDD ‐ Lithodidae Paralomis anamerae Macpherson, 1988 DCP (Spider shrimps) Nematocarcinidae Nematocarcinus longirostris (Bate, 1888) LBT Tristan rock lobster Palinuridae Jasus tristani Holthuis, 1963 PJJ Cape jagged lobster Palinuridae Projasus parkeri (Stebbing, 1902) PDZ Grimald's nylon shrimp Pandalidae Heterocarpus grimaldii Milne‐Edwards & Bouvier, 1900 OCC Common octopus Octopodidae Octopus vulgaris Cuvier, 1797 OFJ Red flying squid Ommastrephidae Ommastrephes bartramii (Lesueur, 1821) OFE Orangeback squid Ommastrephidae Sthenoteuthis pteropus Steenstrup, 1855 Cephalopods SQG Angolan flying -

The Conservation Status of Pelagic Sharks and Rays
The Conservation Status of The Conservation Status of Pelagic Sharks and Rays The Conservation Status of Pelagic Sharks and Rays Pelagic Sharks and Rays Report of the IUCN Shark Specialist Group Pelagic Shark Red List Workshop Report of the IUCN Shark Specialist Group Tubney House, University of Oxford, UK, 19–23 February 2007 Pelagic Shark Red List Workshop Compiled and edited by Tubney House, University of Oxford, UK, 19–23 February 2007 Merry D. Camhi, Sarah V. Valenti, Sonja V. Fordham, Sarah L. Fowler and Claudine Gibson Executive Summary This report describes the results of a thematic Red List Workshop held at the University of Oxford’s Wildlife Conservation Research Unit, UK, in 2007, and incorporates seven years (2000–2007) of effort by a large group of Shark Specialist Group members and other experts to evaluate the conservation status of the world’s pelagic sharks and rays. It is a contribution towards the IUCN Species Survival Commission’s Shark Specialist Group’s “Global Shark Red List Assessment.” The Red List assessments of 64 pelagic elasmobranch species are presented, along with an overview of the fisheries, use, trade, and management affecting their conservation. Pelagic sharks and rays are a relatively small group, representing only about 6% (64 species) of the world’s total chondrichthyan fish species. These include both oceanic and semipelagic species of sharks and rays in all major and Claudine Gibson L. Fowler Sarah Fordham, Sonja V. Valenti, V. Camhi, Sarah Merry D. Compiled and edited by oceans of the world. No chimaeras are known to be pelagic. Experts at the workshop used established criteria and all available information to update and complete global and regional species-specific Red List assessments following IUCN protocols. -

5Th Meeting of the Scientific Committee SC5-DW09 Rev1
5th Meeting of the Scientific Committee Shanghai, China, 23 - 28 September 2017 SC5-DW09_rev1 Ecosystem approach considerations: Deepwater chondrichthyans (sharks, rays and chimaeras) in the Western SPRFMO Area Clinton Duffy1, Shane Geange1 & Tiffany Bock2 1 Department of Conservation 2 Ministry for Primary Industries 1 23 Aug 2017 SC5-DW09_rev1 1. Purpose of paper This paper provides a characterisation of the catch of chondrichthyans in New Zealand bottom fisheries in the SPRFMO Area and information on potential risks to deepwater chondrichthyan species from SPRFMO bottom fishing. Chondrichthyans, particularly those which predominantly occur or complete most of their lifecycle below 200 m depth, are known to have life history characteristics which make them especially vulnerable to fishing pressure. 2. Background About half of chondrichthyans are considered deepwater species, of which around half are sharks (predominantly squaloid dogfishes, Order Squaliformes, and catsharks, Order Carcharhiniformes, Families Pentanchidae and Scyliorhinidae)), with the remainder being skates (predominantly Arhynchobatidae, Rajidae, and Anacanthobatidae), and holocephalans (Kyne & Simpfendorfer 2007). There are currently 177 species reported from the SPRFMO Area that are known to regularly occur below 200 m depth (Appendix 1). Chondrichthyans generally exhibit relatively slow growth rates, late age at maturity, low fecundity and low natural mortality. Knowledge of the growth and reproductive parameters of most deepwater species is generally poor or completely lacking. For the limited number of deepwater species for which sufficient life history data is available, their estimated intrinsic rebound potential values (i.e., ability of a species to recover from fishing pressure) fall at the lower end of the chondrichthyan productivity scale, and include the lowest levels observed (Kyne & Simpfendorfer 2007). -

An Overview of Shark Data Collection by Iccat
SCRS/2001/045 AN OVERVIEW OF SHARK DATA COLLECTION BY ICCAT Papa Kebe1, Victor Restrepo1 and Carlos Palma1 SUMMARY Some species of sharks are taken as by-catch in various fisheries directed at tunas. ICCAT has taken steps to collect Atlantic shark statistics, with emphasis on catch of pelagic species. RÉSUMÉ Certaines espèces de requins sont capturées en tant que prise accessoire de diverses pêcheries qui visent les thons. L'ICCAT a entrepris la collecte de statistiques sur les requins de l'Atlantique, en insistant sur la capture des espèces pélagiques RESUMEN Algunas especies de tiburones se pescan como captura fortuita en numerosas pesquerías dirigidas a los túnidos. ICCAT ha tomado medidas para recopilar estadísticas sobre los tiburones del Atlántico, centrándose sobre todo en las capturas de especies pelágicas. PERIOD PRIOR TO 1994 At the meeting of the Sub-Committee on Statistics in 1991, the delegate of the USSR, noting the importance of pelagic sharks in the incidental catches of tuna fisheries and the repercussions that this could have on the shark stocks and on the oceanic ecosystems, proposed that ICCAT collect statistics relating to sharks. The Sub-Committee on Statistics concluded that, bearing in mind the terms of reference of the ICCAT Convention (see Appendix 1), this did not enter into the mandate of the Commission. Nevertheless, the Sub-Committee considered that, given the prestige and reputation of ICCAT in relation to scientific research, and its experience in data base management, ICCAT should take the first steps towards estimating the level of by-catch in the fisheries. It was proposed that this item be included on the agenda of the next meeting of the SCRS, and asked the Secretariat to draw up a questionnaire on these fisheries.