Status of Shark Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bibliography Database of Living/Fossil Sharks, Rays and Chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the Year 2016
www.shark-references.com Version 13.01.2017 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2016 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 13.01.2017 Abstract: This paper contains a collection of 803 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2016. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2016, list of descriptions of extinct and extant species from 2016, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide information on the geographic and depth distribution of newly described species, i.e. the type specimens from the year 1990- 2016 in a hot spot analysis. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, -

Extinction Risk and Conservation of the World's Sharks and Rays
RESEARCH ARTICLE elife.elifesciences.org Extinction risk and conservation of the world’s sharks and rays Nicholas K Dulvy1,2*, Sarah L Fowler3, John A Musick4, Rachel D Cavanagh5, Peter M Kyne6, Lucy R Harrison1,2, John K Carlson7, Lindsay NK Davidson1,2, Sonja V Fordham8, Malcolm P Francis9, Caroline M Pollock10, Colin A Simpfendorfer11,12, George H Burgess13, Kent E Carpenter14,15, Leonard JV Compagno16, David A Ebert17, Claudine Gibson3, Michelle R Heupel18, Suzanne R Livingstone19, Jonnell C Sanciangco14,15, John D Stevens20, Sarah Valenti3, William T White20 1IUCN Species Survival Commission Shark Specialist Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 2Earth to Ocean Research Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 3IUCN Species Survival Commission Shark Specialist Group, NatureBureau International, Newbury, United Kingdom; 4Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, United States; 5British Antarctic Survey, Natural Environment Research Council, Cambridge, United Kingdom; 6Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, Australia; 7Southeast Fisheries Science Center, NOAA/National Marine Fisheries Service, Panama City, United States; 8Shark Advocates International, The Ocean Foundation, Washington, DC, United States; 9National Institute of Water and Atmospheric Research, Wellington, New Zealand; 10Global Species Programme, International Union for the Conservation -

An Introduction to the Classification of Elasmobranchs
An introduction to the classification of elasmobranchs 17 Rekha J. Nair and P.U Zacharia Central Marine Fisheries Research Institute, Kochi-682 018 Introduction eyed, stomachless, deep-sea creatures that possess an upper jaw which is fused to its cranium (unlike in sharks). The term Elasmobranchs or chondrichthyans refers to the The great majority of the commercially important species of group of marine organisms with a skeleton made of cartilage. chondrichthyans are elasmobranchs. The latter are named They include sharks, skates, rays and chimaeras. These for their plated gills which communicate to the exterior by organisms are characterised by and differ from their sister 5–7 openings. In total, there are about 869+ extant species group of bony fishes in the characteristics like cartilaginous of elasmobranchs, with about 400+ of those being sharks skeleton, absence of swim bladders and presence of five and the rest skates and rays. Taxonomy is also perhaps to seven pairs of naked gill slits that are not covered by an infamously known for its constant, yet essential, revisions operculum. The chondrichthyans which are placed in Class of the relationships and identity of different organisms. Elasmobranchii are grouped into two main subdivisions Classification of elasmobranchs certainly does not evade this Holocephalii (Chimaeras or ratfishes and elephant fishes) process, and species are sometimes lumped in with other with three families and approximately 37 species inhabiting species, or renamed, or assigned to different families and deep cool waters; and the Elasmobranchii, which is a large, other taxonomic groupings. It is certain, however, that such diverse group (sharks, skates and rays) with representatives revisions will clarify our view of the taxonomy and phylogeny in all types of environments, from fresh waters to the bottom (evolutionary relationships) of elasmobranchs, leading to a of marine trenches and from polar regions to warm tropical better understanding of how these creatures evolved. -

Korea, Republic Of
Ministry for Food, Agriculture, Forestry and Fisheries National Plan of Action for the Conservation and Management of Sharks The Republic of Korea August, 2011 MIFAFF Ministry for Food, Agriculture, Forestry and Fisheries I. Introduction ············································································ 2 II. Current State of Shark Management in Korea ························· 3 2.1. Spatial Distribution of Sharks and Related Fisheries ····················· 3 2.2. Shark Catch Statistics ··································································· 7 2.3. Domestic and International Trade of Sharks ································· 8 2.4. Management of Sharks ································································· 9 III. National Plan of Action for the Conservation and Management of Sharks · 10 3.1. Objectives and Scope of National Plan of Action for Sharks······· 10 3.2. Data Collection, Analysis and Assessment ··································· 11 3.3. Conservation and Management Measures ···································· 13 3.4. Monitoring, Control and Surveillance ··········································· 15 3.5. Maximizing the Utilization of Sharks Caught ······························· 16 3.6. Research and Development ························································· 17 3.7. Public Information and Promotion ··············································· 18 3.8. International Cooperation ····························································· 19 3.9. Other Issues ················································································ -

Reproductive Biology of the Bonnethead (Sphyrna Tiburo) from the Southeastern U.S
University of North Florida UNF Digital Commons UNF Graduate Theses and Dissertations Student Scholarship 2014 Reproductive Biology of the Bonnethead (Sphyrna tiburo) from the Southeastern U.S. Atlantic Coast Melissa I. Gonzalez De Acevedo University of North Florida, [email protected] Follow this and additional works at: https://digitalcommons.unf.edu/etd Part of the Biology Commons, and the Ecology and Evolutionary Biology Commons Suggested Citation Gonzalez De Acevedo, Melissa I., "Reproductive Biology of the Bonnethead (Sphyrna tiburo) from the Southeastern U.S. Atlantic Coast" (2014). UNF Graduate Theses and Dissertations. 534. https://digitalcommons.unf.edu/etd/534 This Master's Thesis is brought to you for free and open access by the Student Scholarship at UNF Digital Commons. It has been accepted for inclusion in UNF Graduate Theses and Dissertations by an authorized administrator of UNF Digital Commons. For more information, please contact Digital Projects. © 2014 All Rights Reserved REPRODUCTIVE BIOLOGY OF THE BONNETHEAD (SPHYRNA TIBURO) FROM THE SOUTHEASTERN U.S. ATLANTIC COAST by Melissa Gonzalez De Acevedo A thesis submitted to the Department of Biology in partial fulfillment of the requirements for the degree of Masters of Science in Biology UNIVERSITY OF NORTH FLORIDA COLLEGE OF ARTS AND SCIENCES December 2014 Unpublished work, © Melissa Gonzalez De Acevedo CERTIFICATE OF APPROVAL The thesis “Reproductive biology of the bonnethead (Sphyrna tiburo) from the southeastern U.S. Atlantic coast” submitted by Melissa Gonzalez De Acevedo Approved by the thesis committee: Date Dr. Jim Gelsleichter Committee Chair Dr. Carolyn Belcher Dr. Eric Johnson Accepted for the Department of Biology: Dr. Cliff Ross Assistant Chair Accepted for the College of Arts and Sciences: Dr. -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

Florida's Fintastic Sharks and Rays Lesson and Activity Packet
Florida's Fintastic Sharks and Rays An at-home lesson for grades 3-5 Produced by: This educational workbook was produced through the support of the Indian River Lagoon National Estuary Program. 1 What are sharks and rays? Believe it or not, they’re a type of fish! When you think “fish,” you probably picture a trout or tuna, but fishes come in all shapes and sizes. All fishes share the following key characteristics that classify them into this group: Fishes have the simplest of vertebrate hearts with only two chambers- one atrium and one ventricle. The spine in a fish runs down the middle of its back just like ours, making fish vertebrates. All fishes have skeletons, but not all fish skeletons are made out of bones. Some fishes have skeletons made out of cartilage, just like your nose and ears. Fishes are cold-blooded. Cold-blooded animals use their environment to warm up or cool down. Fins help fish swim. Fins come in pairs, like pectoral and pelvic fins or are singular, like caudal or anal fins. Later in this packet, we will look at the different types of fins that fishes have and some of the unique ways they are used. 2 Placoid Ctenoid Ganoid Cycloid Hard protective scales cover the skin of many fish species. Scales can act as “fingerprints” to help identify some fish species. There are several different scale types found in bony fishes, including cycloid (round), ganoid (rectangular or diamond), and ctenoid (scalloped). Cartilaginous fishes have dermal denticles (Placoid) that resemble tiny teeth on their skin. -

Order LAMNIFORMES ODONTASPIDIDAE Sand Tiger Sharks Iagnostic Characters: Large Sharks
click for previous page Lamniformes: Odontaspididae 419 Order LAMNIFORMES ODONTASPIDIDAE Sand tiger sharks iagnostic characters: Large sharks. Head with 5 medium-sized gill slits, all in front of pectoral-fin bases, Dtheir upper ends not extending onto dorsal surface of head; eyes small or moderately large, with- out nictitating eyelids; no nasal barbels or nasoral grooves; snout conical or moderately depressed, not blade-like;mouth very long and angular, extending well behind eyes when jaws are not protruded;lower labial furrows present at mouth corners; anterior teeth enlarged, with long, narrow, sharp-edged but unserrated cusps and small basal cusplets (absent in young of at least 1 species), the upper anteriors separated from the laterals by a gap and tiny intermediate teeth; gill arches without rakers; spiracles present but very small. Two moderately large high dorsal fins, the first dorsal fin originating well in advance of the pelvic fins, the second dorsal fin as large as or somewhat smaller than the first dorsal fin;anal fin as large as second dorsal fin or slightly smaller; caudal fin short, asymmetrical, with a strong subterminal notch and a short but well marked ventral lobe. Caudal peduncle not depressed, without keels; a deep upper precaudal pit present but no lower pit. Intestinal valve of ring type, with turns closely packed like a stack of washers. Colour: grey or grey-brown to blackish above, blackish to light grey or white, with round or oval dark spots and blotches vari- ably present on 2 species. high dorsal fins upper precaudal eyes without pit present nictitating eyelids intestinal valve of ring type Habitat, biology, and fisheries: Wide-ranging, tropical to cool-temperate sharks, found inshore and down to moderate depths on the edge of the continental shelves and around some oceanic islands, and in the open ocean. -

Download the Full Article As Pdf ⬇︎
LocalLockdown Diving — Dives Found in Contributors' Backyards Text and photos by Andrey Bizyukin, Larry Cohen, Brent Durand, Dmitry Efremychev, Jennifer Idol, Kate Jonker, Matthew Meier, Pete Mesley, Don Silcock, Olga Torrey and Martin Voeller As many divers face travel restrictions during the coronavi- rus pandemic, our contributors highlight the often overlooked or unsung yet intriguing div- ing that can be found in one's own backyard. X-Ray Mag contributors share their favorite local haunts—from a spring-fed Texan lake to a quarry and a sinkhole in Russia to the tem- perate waters off New Zealand, Japan, South Africa, New Jersey and Northern California to the subtropical waters of Southern California and Sydney, Australia—where they captured compelling underwater images. 58 X-RAY MAG : 101 : 2020 EDITORIAL FEATURES TRAVEL NEWS WRECKS EQUIPMENT BOOKS SCIENCE & ECOLOGY TECH EDUCATION PROFILES PHOTO & VIDEO PORTFOLIO MATTHEW MEIER feature Local Dives School of opaleye and garibaldi among sea grass and feather boa kelp. PREVIOUS PAGE: School of juvenile senorita fish in the kelp and sea grass beds Bat Ray Cove, San Clemente Island, Soupfin (tope) sharks can be seen swimming in California, USA the shallows and among the giant kelp, along with schools of blacksmith and jack mackerels. Text and photos by Matthew Meier California sea lions will swoop through intermittently, and the occasional harbor seal Thankfully, local diving is still possible during will play peak-a-boo in the kelp. the pandemic, and while this dive site requires Under the boat is a sandy bottom where boat access, it is still one of my favorites. -
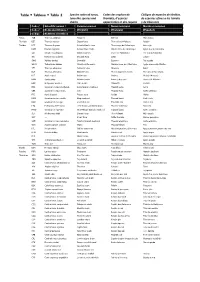
Table Tableau Tabla 2
Table Tableau Tabla 2 Species codes of tunas, Codes des espèces de Códigos de especies de túnidos, tuna‐like species and thonidés, d’espèces de especies afines a los túnidos sharks apparentées et des requins y de tiburones Code / Scientific names / Common names Noms communs Nombres comunes Code / Noms sientifiques / (English) (Français) (Español) Código Nombres científicos Tunas ALB Thunnus alalunga Albacore Germon Atún blanco Thonidés BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo) Patudo Túnidos BFT Thunnus thynnus Atlantic bluefin tuna Thon rouge de l’atlantique Atún rojo BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico SKJ Katsuwonus pelamis Skipjack tuna Listao Listado SWO Xiphias gladius Swordfish Espadon Pez espada WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico YFT Thunnus albacares Yellowfin tuna Albacore Rabil BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras BLT Auxis rochei Bullet tuna Bonitou Melva(=Melvera) BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del Atlántico BOP Orcynopsis unicolor Plain bonito Palomette Tasarte BRS Scomberomorus brasiliensis Serra Spanish mackerel Thazard serra Serra CER Scomberomorus regalis Cero Thazard franc Carite chinigua FRI Auxis thazard Frigate tuna Auxide Melva KGM Scomberomorus cavalla King mackerel Thazard barré Carite lucio KGX Scomberomorus spp -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught. -

An Overview of the Elasmobranch By-Catch of the Queensland East Coast Trawl Fishery (Australia) (Elasmobranch Fisheries – Oral)
NOT TO BE CITED WITHOUT PRIOR REFERENCE TO THE AUTHOR(S) Northwest Atlantic Fisheries Organization Serial No. N4718 NAFO SCR Doc. 02/97 SCIENTIFIC COUNCIL MEETING – SEPTEMBER 2002 An Overview of the Elasmobranch By-catch of the Queensland East Coast Trawl Fishery (Australia) (Elasmobranch Fisheries – Oral) by P. M. Kynea, A.J. Courtneyb, M.J. Campbellb, K.E. Chilcottb, S.W. Gaddesb, C.T. Turnbullc, C.C. Van Der Geestc and M. B. Bennetta a Department of Anatomy and Developmental Biology, The University of Queensland, Brisbane, 4072, Queensland, Australia b Southern Fisheries Centre, Queensland Department of Primary Industries, PO Box 76, Deception Bay, 4508, Queensland, Australia c Northern Fisheries Centre, Queensland Department of Primary Industries, PO Box 5396, Cairns, 4870, Queensland, Australia E-mail: [email protected] Abstract The Queensland East Coast Trawl Fishery (ETCF) is a complex multi-species and multi-sector fishery operating along Queensland’s eastern coastline, with combined annual landings of close to 10 000 tons. Elasmobranchs represent a relatively small, but potentially ecologically significant component of by-catch in this fishery. At least 94 species of elasmobranchs occur in the managed area of the ECTF and a study has been initiated to examine elasmobranch by-catch in four sectors of the fishery, as part of a larger Queensland Department of Primary Industries by-catch project. A total of 42 elasmobranch and one holocephalan species have been recorded as by- catch in the fishery. Preliminary results from fishery-independent (FI) surveys indicate that elasmobranch by-catch is highly variable between fishery sectors. Elasmobranch by-catch is extremely low in the tiger/Endeavour prawn sector, low in the eastern king prawn – deep water sector (EKP-D), and moderate in the EKP – shallow water sector (EKP-S).