Identification Guide to the Deep-Sea Cartilaginous Fishes of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bibliography Database of Living/Fossil Sharks, Rays and Chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the Year 2016
www.shark-references.com Version 13.01.2017 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2016 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 13.01.2017 Abstract: This paper contains a collection of 803 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2016. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2016, list of descriptions of extinct and extant species from 2016, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide information on the geographic and depth distribution of newly described species, i.e. the type specimens from the year 1990- 2016 in a hot spot analysis. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, -

Sharks in Crisis: a Call to Action for the Mediterranean
REPORT 2019 SHARKS IN CRISIS: A CALL TO ACTION FOR THE MEDITERRANEAN WWF Sharks in the Mediterranean 2019 | 1 fp SECTION 1 ACKNOWLEDGEMENTS Written and edited by WWF Mediterranean Marine Initiative / Evan Jeffries (www.swim2birds.co.uk), based on data contained in: Bartolí, A., Polti, S., Niedermüller, S.K. & García, R. 2018. Sharks in the Mediterranean: A review of the literature on the current state of scientific knowledge, conservation measures and management policies and instruments. Design by Catherine Perry (www.swim2birds.co.uk) Front cover photo: Blue shark (Prionace glauca) © Joost van Uffelen / WWF References and sources are available online at www.wwfmmi.org Published in July 2019 by WWF – World Wide Fund For Nature Any reproduction in full or in part must mention the title and credit the WWF Mediterranean Marine Initiative as the copyright owner. © Text 2019 WWF. All rights reserved. Our thanks go to the following people for their invaluable comments and contributions to this report: Fabrizio Serena, Monica Barone, Adi Barash (M.E.C.O.), Ioannis Giovos (iSea), Pamela Mason (SharkLab Malta), Ali Hood (Sharktrust), Matthieu Lapinksi (AILERONS association), Sandrine Polti, Alex Bartoli, Raul Garcia, Alessandro Buzzi, Giulia Prato, Jose Luis Garcia Varas, Ayse Oruc, Danijel Kanski, Antigoni Foutsi, Théa Jacob, Sofiane Mahjoub, Sarah Fagnani, Heike Zidowitz, Philipp Kanstinger, Andy Cornish and Marco Costantini. Special acknowledgements go to WWF-Spain for funding this report. KEY CONTACTS Giuseppe Di Carlo Director WWF Mediterranean Marine Initiative Email: [email protected] Simone Niedermueller Mediterranean Shark expert Email: [email protected] Stefania Campogianni Communications manager WWF Mediterranean Marine Initiative Email: [email protected] WWF is one of the world’s largest and most respected independent conservation organizations, with more than 5 million supporters and a global network active in over 100 countries. -

Extinction Risk and Conservation of the World's Sharks and Rays
RESEARCH ARTICLE elife.elifesciences.org Extinction risk and conservation of the world’s sharks and rays Nicholas K Dulvy1,2*, Sarah L Fowler3, John A Musick4, Rachel D Cavanagh5, Peter M Kyne6, Lucy R Harrison1,2, John K Carlson7, Lindsay NK Davidson1,2, Sonja V Fordham8, Malcolm P Francis9, Caroline M Pollock10, Colin A Simpfendorfer11,12, George H Burgess13, Kent E Carpenter14,15, Leonard JV Compagno16, David A Ebert17, Claudine Gibson3, Michelle R Heupel18, Suzanne R Livingstone19, Jonnell C Sanciangco14,15, John D Stevens20, Sarah Valenti3, William T White20 1IUCN Species Survival Commission Shark Specialist Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 2Earth to Ocean Research Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 3IUCN Species Survival Commission Shark Specialist Group, NatureBureau International, Newbury, United Kingdom; 4Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, United States; 5British Antarctic Survey, Natural Environment Research Council, Cambridge, United Kingdom; 6Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, Australia; 7Southeast Fisheries Science Center, NOAA/National Marine Fisheries Service, Panama City, United States; 8Shark Advocates International, The Ocean Foundation, Washington, DC, United States; 9National Institute of Water and Atmospheric Research, Wellington, New Zealand; 10Global Species Programme, International Union for the Conservation -

Molecular Circumscription of New Species of Gyrocotyle Diesing, 1850 (Cestoda) from Deep-Sea Chimaeriform Holocephalans in the North Atlantic
Title Molecular circumscription of new species of Gyrocotyle Diesing, 1850 (Cestoda) from deep-sea chimaeriform holocephalans in the North Atlantic Authors Bray, RA; Waeschenbach, A; Littlewood, T; Halvorsen, O; Olson, PD Date Submitted 2020-06-11 Syst Parasitol https://doi.org/10.1007/s11230-020-09912-w (0123456789().,-volV)(0123456789().,-volV) Molecular circumscription of new species of Gyrocotyle Diesing, 1850 (Cestoda) from deep-sea chimaeriform holocephalans in the North Atlantic Rodney A. Bray . Andrea Waeschenbach . D. Timothy J. Littlewood . Odd Halvorsen . Peter D. Olson Received: 14 November 2019 / Accepted: 1 March 2020 Ó The Author(s) 2020 Abstract Chimaeras, or ratfishes, are the only extant and their specific host associations has remained group of holocephalan fishes and are the sole host highly speculative. Here we report the presence of group of gyrocotylidean cestodes, which represent a Gyrocotyle spp. from rarely-caught deep-sea chi- sister group of the true tapeworms (Eucestoda). These maeras collected in the North-East Atlantic, and unique, non-segmented cestodes have been known describe two new species: G. haffii n. sp. from the since the 1850s and multiple species and genera have bent-nose chimaera, Harriota raleighana Goode & been erected despite a general agreement that the Bean, and G. discoveryi n. sp. from the large-eyed delineation of species on the basis of morphology is rabbit fish, Hydrolagus mirabilis (Collett). Nuclear effectively impossible. Thus, in the absence of ribosomal sequence data were generated for individual molecular studies, the validity of gyrocotylid taxa parasites taken from different host species collected on different dates and from different localities and were combined with previously published sequences. -

AC26 Inf. 1 (English Only / Únicamente En Inglés / Seulement En Anglais)
AC26 Inf. 1 (English only / únicamente en inglés / seulement en anglais) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-sixth meeting of the Animals Committee Geneva (Switzerland), 15-20 March 2012 and Dublin (Ireland), 22-24 March 2012 RESPONSE TO NOTIFICATION TO THE PARTIES NO. 2011/049, CONCERNING SHARKS The attached information document has been submitted by the Secretariat at the request of PEW, in relation to agenda item 16*. * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat or the United Nations Environment Programme concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. AC26 Inf. 1 – p. 1 January 5, 2012 Pew Environment Group Response to CITES Notification 2011/049 To Whom it May Concern, As an active international observer to CITES, a member of the Animals Committee Shark Working Group, as well as other working groups of the Animals and Standing Committees, and an organization that is very active in global shark conservation, the Pew Environment Group submits the following information in response to CITES Notification 2011/049. We submit this information in an effort to ensure a more complete response to the request for information, especially considering that some countries that have adopted proactive new shark conservation policies are not Parties to CITES. 1. Shark species which require additional action In response to Section a) ii) of the Notification, the Pew Environment Group submits the following list of shark species requiring additional action to enhance their conservation and management. -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

The Shark Tagger 1992 Summary
THE SHARK TAGGER 1992 SUMMARY ' Newsletter of the Cooperative Shark Tagging Program PHOTO BY H. W. PRATT In this issue: • 1992 A Milestone Year - 100,000th Shark Tagged • Record Number of Sharks Tagged (8451) and Recaptured (506) in 1992 • Sandbar Shark Recaptured after 24.9 Years • Second Trans-Atlantic Recapture of a Mako Shark -la ~ • Tagged Blue Shark Crosses Equator ~ Q N.M.F.S. (j(j Vo,.1.. 111~ • Porbeagle Sharks Recaptured after 4, 6, and 8 l'' SHARI.'- Years • Record Distance for Bignose Shark - 1800 1992 Overview Miles - New Jersey to Texas The number offish released (8451) and returned (506) In • 38% of Recaptured Blue Sharks Released Again 1992 ls the highest for any year In the three decade long Cooperative Shark Tagging Program. In addition, the one • Pregnant Mako Shark with 14 Pups Examined hundred thousandth shark was tagged In 1992 adding another mtlestone. Identification of that particular shark and acknowledgement of the individual tagger would be Approximately 400 new taggers Joined the Tagging Pro- dtfficult as hundreds of tags are used along the U.S. East gram this year. We arc Including a brief overview oftaggtng Coast on any particular day. Recognition for this outstand- studies and the results to date of our Tagging Program to Ing accomplishment deservedly goes to every member of the familiarize new members with our research. Program who ever put a tag In a fish. In addition to the Tagging studies are useful to characterize the sharks In thousandsofcooperatlngftshermenandsclentlsts, wewould an area In terms of species. sex, and size; to help define Uketoacknowledgethemanysportclubs. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

On the Occurrence of the Arrowhead Dogfish, Deania Profundorum
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sapientia On the occurrence of the arrowhead dogfish, Deania profundorum (Chondrichthyes: Squalidae) off southern Portugal, with a missing gill slit by Rui COELHO & Karim ERZINI (1) R É S U M É. - Signalement d’un Deania pro f u n d o ru m ( C h o n d r i c h- thyes : Squalidae) capturé dans le sud du Portugal, avec absence d’une fente branchiale. Dans ce travail, nous rapportons la capture d’un chien de mer pointe de flèche, Deania pro f u n d o ru m (Smith & Radcliffe, 1912), dans les eaux portugaises méridionales. Le spécimen, une grande femelle mature de 87,5 cm de longueur totale, n’avait que quatre fentes branchiales du côté droit, sans présenter de cicatrice à l’en- droit où la cinquième fente aurait dû se situer. Des mesures compa- ratives entre les tailles des fentes branchiales gauches et droites amènent à conclure que la fente manquante est probablement la première. Key words. - Chondrichthyes - Squalidae - Deania pro f u n d o ru m - ANE - Southern Portugal - Gill slit deformation - Record. The arrowhead dogfish, Deania pro f u n d o ru m (Smith & Rad- cliffe, 1912), is a squalid shark characterized by a greatly elongated snout, that is spatulate dorsal-ventrally and thin-depressed laterally (Compagno, 1984). This is a widely distributed species found on Figure 1. - Map of the southwest coast of Portugal with location of the cap- both sides of the Atlantic, from the Western Sahara to South A f r i c a ture ( ) of the Deania pro f u n d o ru m specimen. -
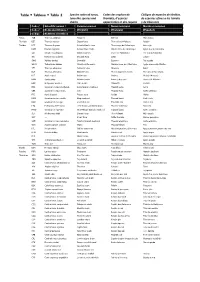
Table Tableau Tabla 2
Table Tableau Tabla 2 Species codes of tunas, Codes des espèces de Códigos de especies de túnidos, tuna‐like species and thonidés, d’espèces de especies afines a los túnidos sharks apparentées et des requins y de tiburones Code / Scientific names / Common names Noms communs Nombres comunes Code / Noms sientifiques / (English) (Français) (Español) Código Nombres científicos Tunas ALB Thunnus alalunga Albacore Germon Atún blanco Thonidés BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo) Patudo Túnidos BFT Thunnus thynnus Atlantic bluefin tuna Thon rouge de l’atlantique Atún rojo BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico SKJ Katsuwonus pelamis Skipjack tuna Listao Listado SWO Xiphias gladius Swordfish Espadon Pez espada WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico YFT Thunnus albacares Yellowfin tuna Albacore Rabil BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras BLT Auxis rochei Bullet tuna Bonitou Melva(=Melvera) BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del Atlántico BOP Orcynopsis unicolor Plain bonito Palomette Tasarte BRS Scomberomorus brasiliensis Serra Spanish mackerel Thazard serra Serra CER Scomberomorus regalis Cero Thazard franc Carite chinigua FRI Auxis thazard Frigate tuna Auxide Melva KGM Scomberomorus cavalla King mackerel Thazard barré Carite lucio KGX Scomberomorus spp -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught. -

Wk Shark Advice Adhshark
ICES Special Request Advice Ecoregions in the Northeast Atlantic and adjacent seas Published 25 September 2020 NEAFC and OSPAR joint request on the status and distribution of deep-water elasmobranchs Advice summary In response to a joint request from NEAFC and OSPAR, ICES reviewed existing information on deep-water sharks, skates and rays from surveys and the literature. Distribution maps were generated for 21 species, showing the location of catches from available survey data on deep-water sharks and elasmobranchs in the NEAFC and OSPAR areas of the Northeast Atlantic. Shapefiles of the species distribution areas are available as supporting documentation to this work. This advice sheet presents a summary of ICES advice on the stock status of species for which an assessment is available, as well as current knowledge on the stock status of species for which ICES does not provide advice. An overview of approaches which may be applied to mitigate bycatch and to improve stock status is also presented. ICES recognizes that, despite their limitations, prohibition, gear and depth limitations, and TAC are mechanisms currently available to managers to regulate outtake; therefore, ICES advises that these mechanisms should be maintained. Furthermore, ICES advises that additional measures, such as electromagnetic exclusion devices, acoustic or light-based deterrents, and spatio-temporal management could be explored. Request NEAFC and OSPAR requested ICES to produce: a. Maps and shapefiles of the distribution of the species, identifying, if possible, key areas used during particular periods/stages of the species’ lifecycle in terms of distribution and relative abundance of the species, and expert interpretation of the data products; b.