APPENDIX D – Provisional SEAFO Species List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ontogeny of Photophore Pattern in the Velvet Belly Lantern Shark, Etmopterus Spinax Julien M
ARTICLE IN PRESS ZOOLOGY Zoology 112 (2009) 433–441 www.elsevier.de/zool Ontogeny of photophore pattern in the velvet belly lantern shark, Etmopterus spinax Julien M. ClaesÃ,Je´roˆme Mallefet Catholic University of Louvain, Laboratory of Marine Biology, Place Croix du Sud, Kellner Building, B-1348, Louvain-la-Neuve, Belgium Received 8 October 2008; received in revised form 23 January 2009; accepted 23 February 2009 Abstract Bioluminescence is known to be of great ecological importance to a luminous organism but extremely few studies investigate the ontogeny of luminous capabilities. The photogenic pattern of the velvet belly lantern shark Etmopterus spinax was investigated over ontogeny (14.0–52.5 cm total length) to determine the scaling of the surface area and the photophore density of different luminous zones as well as the ecological consequences of ontogenetic variations in bioluminescence efficiency. According to the luminous zone considered, different scaling patterns were found for the surface areas while the photophore densities of all zones scale with negative allometry, even though photophore insertion occurs. No sexual differences in these relationships were found. Luminous zones can be placed in two morphologically different groups: the ‘‘coverage’’ and the ‘‘isolated’’ zones. While counter-illumination is certainly the function of the former, the latter are probably involved in intraspecific behaviours. Due to the discrepancy between luminous capabilities of these two luminous zone categories, there is an ontogenetic increase in the luminescence heterogeneity of the luminous pattern as it was shown by luminescence modelling and confirmed by direct observations of spontaneous luminescence in living sharks. This heterogeneity certainly represents a trade-off between an efficient ventral camouflage and a strong identification tool for intraspecific behaviours such as coordinate hunting, which would be particularly useful when E. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Atlas of Marine Bony Fish Otoliths (Sagittae) of Southeastern-Southern
Original Article / Artigo Original Conversani et al.: Sagittae from the SouthwestBJOCE Atlantic Ocean Atlas of marine bony fish otoliths (sagittae) of Southeastern-Southern Brazil Part VII: Atheriniformes, Beloniformes, Beryciformes, Zeiformes, Syngnathiformes, Scorpaeniformes and Tetraodontiformes Valéria Regina Martins Conversani1, Marina Rito Brenha-Nunes1, César Santificetur1, Marcella Bockis Giaretta1, Carolina Correia Siliprandi1, Carmen Lucia Del Bianco Rossi-Wongtschowski1* 1 Instituto Oceanográfico da Universidade de São Paulo (Praça do Oceanográfico, 191, 05508-120 São Paulo, SP, Brazil) *Corresponding author: [email protected] ABSTRACT RESUMO In addition to the series of documents that we have Em adição à série de documentos que estamos been publishing on the "Atlas of Teleostei Otoliths publicando sobre o "Atlas de Otólitos para os peixes for the Southeastern-Southern Brazilian region", in Teleósteos da região Sudeste-Sul do Brasil", neste this volume we present the results of species of the volume apresentamos os resultados obtidos para espécies orders Atheriniformes (1 species), Beloniformes (5), das ordens Atheriniformes (1 espécie), Beloniformes Beryciformes (2), Zeiformes (2), Syngnathiformes (5), Beryciformes (2), Zeiformes (2), Syngnathiformes (2), Scorpaeniformes (9) and Tetraodontiformes (6). (2), Scorpaeniformes (9) e Tetraodontiformes (6). Foram Features, measurements and indices were analyzed analisadas as feições, medidas e índices usualmente according to methodology used in anterior series. empregados conforme metodologia -

Expert Consultation on Deep-Sea Fisheries in the High Seas
FAO Fisheries Report No. 838 FIEP/R838 (En) ISSN 0429-9337 FAO/JAPAN GOVERNMENT COOPERATIVE PROGRAMME GCP/INT/942/JPN Report and documentation of the EXPERT CONSULTATION ON DEEP-SEA FISHERIES IN THE HIGH SEAS Bangkok, Thailand, 21–23 November 2006 Cover photo: Image of rusky knoll, a deep sea feature in the Indian Ocean. Courtesy of Graham Patchell, Sealord Grp, New Zealand. This knoll is now closed to bottom trawling largely due to the presence of black coral. The red lines indicate known tow lines. The majority of the tows have come close to the top of the knoll and landed below the ledge at 690 metres which surrounds the knoll. Copies of FAO publications can be requested from: Sales and Marketing Group Communication Division FAO Viale delle Terme di Caracalla 00153 Rome, Italy E-mail: [email protected] Fax: +39 06 57053360 Web site: http://www.fao.org FAO Fisheries Report No. 838 FIEP/R838 (En) Report and documentation of the EXPERT CONSULTATION ON DEEP-SEA FISHERIES IN THE HIGH SEAS Bangkok, Thailand, 21–23 November 2006 FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2007 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught. -

Centriscidae Bonaparte, 1831 - Razorfishes, Shrimpfishes
FAMILY Centriscidae Bonaparte, 1831 - razorfishes, shrimpfishes SUBFAMILY Macroramphosinae Bleeker, 1879 - razorfishes, snipefishes [=Siluridi, Orthichthyinae, Macrorhamphoidei] Notes: Name in prevailing recent practice Siluridi Rafinesque, 1810b:35 [ref. 3595] (ordine) Macroramphosus [no stem of the type genus, no available, Article 11.7.1.1] Orthichthyinae Gill, 1862j:234 (footnote) [ref. 1663] (subfamily) Orthichthys [family-group name never used as valid after 1899] Macrorhamphoidei Bleeker, 1879a:14 [ref. 460] (family) Macroramphosus [Macrorhamphus inferred from the stem, Article 11.7.1.1; name must be corrected Article 32.5.3; stem corrected to Macroramphos- by Gill 1884c:156, 162 [ref. 17660]; family-group name used as valid by: Schultz with Stern 1948 [ref. 31938], Kamohara 1967, McAllister 1968 [ref. 26854], Lindberg 1971 [ref. 27211], Lagler, Bardach, Miller & May Passino 1977, Nelson 1984 [ref. 13596], Smith & Heemstra 1986 [ref. 5715], Whitehead et al. (1986a) [ref. 13676], Paxton et al. 1989 [ref. 12442], Quéro et al. 1990 [ref. 15946], Nelson 1994 [ref. 26204], Eschmeyer 1998 [ref. 23416], Menezes et al. 2003 [ref. 27192], Nelson et al. 2004 [ref. 27807], Hoese et al. 2006, Nelson 2006 [ref. 32486]; family name sometimes seen as †Rhamphosidae] GENUS Centriscops Gill, 1862 - bellowsfishes [=Centriscops Gill [T. N.], 1862:234, Limiculina (subgenus of Macrorhamphosus) Fowler [H. W.], 1907:425] Notes: [ref. 1663]. Masc. Centriscus humerosus Richardson, 1846. Type by monotypy. •Valid as Centriscops Gill, 1862 -- (Mohr 1937:53 [ref. 15290], Heemstra 1986:459 [ref. 5660], Paxton et al. 1989:407 [ref. 12442], Duhamel 1995:264 [ref. 21927], Gomon et al. 1994:436 [ref. 22532], Keivany & Nelson 2006:S84 [ref. 28978], Paxton et al. -

Coelho Phd Lantern S
UNIVERSIDADEdo ALGARVE FaculdadedeCiênciasdoMaredo Ambiente Biology,populationdynamics,managementandconservation ofdeepwaterlanternsharks,Etmopterusspinax and Etmopteruspusillus (Chondrichthyes:Etmopteridae)insouthernPortugal(northeastAtlantic). (DoutoramentoemCiênciaseTecnologiasdasPescas,especialidadedeBiologiaPesqueira) (ThesisforthedegreeinDoctorofPhilosophyinFisheriesSciencesandTechnologies,specialtyinFisheriesBiology) RUIPEDROANDRADECOELHO Faro (2007) UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS DO MAR E DO AMBIENTE Biology, population dynamics, management and conservation of deep water lantern sharks, Etmopterus spinax and Etmopterus pusillus (Chondrichthyes: Etmopteridae) in southern Portugal (northeast Atlantic). (Doutoramento em Ciências e Tecnologias das Pescas, especialidade de Biologia Pesqueira) (Thesis for the degree in Doctor of Philosophy in Fisheries Sciences and Technologies, specialty in Fisheries Biology) RUI PEDRO ANDRADE COELHO Orientador / Supervisor: Prof. Doutor Karim Erzini Júri / Jury: - Prof. Doutor José Pedro Andrade, Professor Catedrático da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Karim Erzini, Professor Associado com Agregação da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Leonel Paulo Sul de Serrano Gordo, Professor Auxiliar com Agregação da Faculdade de Ciências, Universidade de Lisboa; - Prof. Doutor Manuel Seixas Afonso Dias, Professor Auxiliar da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; -

Seamounts, Hotspots for High Speciation Rates in Bentho-Pelagic Fishes? a Case Study on Macroramphosus Spp
Matthiessen et al., Macroramphosus CM 2002/M: 07 1 Ices CM 2002 / Oceanography and Ecology of Seamounts - Indications of Unique Ecosystems (Session M) Not to be cited without prior reference to the author. Seamounts, hotspots for high speciation rates in bentho-pelagic fishes? A case study on Macroramphosus spp. (Syngnathidae) from Great Meteor Seamount BIRTE MATTHIESSEN, HEINO O. FOCK, HEIN VON WESTERNHAGEN Alfred-Wegener-Institut für Polar- und Meeresforschung, Am Handelshafen 12, D-27570 Bremerhaven, Germany, Phone: 49-0471-4831 1384, [email protected] Snipefishes of the genus Macroramphosus (Syngnathidae) are the most abundant demersal fish species on the Great Meteor Seamount (GMR, subtropical NE Atlantic, 30° N, 28,5° W). High morphological variability within the genus at GMR questions the presence of only one species of Macroramphosus. Thus, 202 individuals of Macroramphosus spp. were investigated with respect to diet composition and morphology. A deep-bodied benthos feeding type (b-types) whose diet consisted of foraminiferans, pteropods, decapods and polychaetes, could be distinguished from a slender planktivorous type (p-types, n=140) mainly feeding on ostracods, copepods, pteropods and foraminiferans. Few specimens showed no specialized feeding mode (p/b-types). Both feeding types showed significantly morphologically differences after applying bi- and multivariate statistical analysis considering the variables body depth, length of second dorsal spine, diameter of orbit and standard length. Based on a randomisation test, the analysis of dietary overlaps revealed significant differences between feeding types. This result reinforces the hypothesis of two competing species of snipefishes at GMR. Notwithstanding similar observations on snipefishes from shelf habitats from the Pacific, our results suggest that isolated seamounts may represent hotspots of speciation where special selection pressure favours the evolution of two ecological divergent species within the genus Macroramphosus. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -
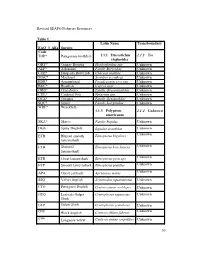
Revised SEAFO Fisheries Resources Table 1. . FAO 3 Alfa Species Code
Revised SEAFO Fisheries Resources Table 1. Latin Name Transboundary FAO 3 Alfa Species Code TOP* Patagonian toothfish 1.1.1 Dissostichus 1.1.2 Yes eleginoides ORY* Orange Roughy Hoplosthethus spp Unknown ALF* Alfonsino Family Berycidae Unknown CGE* Deep-sea Red Crab Chaceon maritae Unknown MAC* Mackerel Scomber scombrus Unknown EDR* Armourhead Pseudopentaceros spp. Unknown BOC* Boarfish Capros aper Unknown ORD* Oreo dories Family Oreosomatidae Unknown CDL* Cardinal Fish Epigonus spp. Unknown OCZ* Octopus Family Octopodidae Unknown SQC* Squid Family Loliginidae Unknown WRF* Wreckfish 1.1.3 Polyprion 1.1.4 Unknown americanus SKA* Skates Family Rajidae Unknown DGS Spiny Dogfish Squalus acanthias Unknown Unknown ETB Blurred smooth Etmopterus bigelowi lanternshark ETH Shorttail Etmopterus brachyurus Unknown lanternshark ETR Great lanternshark Etmopterus princeps Unknown ETP Smooth lanternshark Etmopterus pusillus Unknown Unknown APA Ghost catshark Apristurus manis SSQ Velvet dogfish Scymnodon squamulosus Unknown CYO Portuguese Dogfish Centroscymnus coelolepis Unknown GUQ Leafscale Gulper Centrophorus squamosus Unknown Shark GUP Gulper Shark Centrophorus granulosus Unknown CFB ǂ Black dogfish Centroscyllium fabricii Unknown CYP ǂ Longnose velvet Centroscymnus crepidater Unknown 30 dogfish CYY Unknown ǂ Shortnose velvet Centroscymnus dogfish cryptacanthus SCK ǂ Kitefin shark Dalatias licha Unknown ETE ǂ Etmopterus compagnoi Unknown ETI ǂ Broadbanded Etmopterus gracilispinis Unknown lanternshark ETM ǂ Unknown Southern Etmopterus granulosus lanternshark