Seribantumab Poster AACR 03.28.21
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lung Cancer Drugs in the Pipeline
HemOnc today | JANUARY 10, 2016 | Healio.com/HemOnc 5 Lung Cancer Drugs in the Pipeline HEMONC TODAY presents this guide to drugs in phase 2 or phase 3 development for lung cancer-related indications. Clinicians can use this chart as a quick reference to learn about the status of those drugs that may be clinically significant to their practice. Generic name (Brand name, Manufacturer) Indication(s) Development status abemaciclib (Eli Lilly) non–small cell lung cancer phase 3 ABP 215 (Allergan/Amgen) non–small cell lung cancer (advanced disease) phase 3 ACP-196 (Acerta Pharma) non–small cell lung cancer (advanced disease) phase 2 ado-trastuzumab emtansine (Kadcyla, Genentech) non–small cell lung cancer (HER-2–positive disease) phase 2 afatinib (Gilotrif, Boehringer Ingelheim) lung cancer (squamous cell carcinoma) phase 3 aldoxorubicin (CytRx) small cell lung cancer phase 2 alectinib (Alecensa, Genentech) non–small cell lung cancer (second-line treatment of ALK-positive disease) phase 2 non–small cell lung cancer (first-line treatment of ALK-positive disease); phase 3 alisertib (Takeda) malignant mesothelioma, small cell lung cancer phase 2 avelumab (EMD Serono/Pfizer) non–small cell lung cancer phase 3 AZD9291 (AstraZeneca) non–small cell lung cancer (first-line treatment of advancedEGFR -positive disease; phase 3 second-line treatment of advanced EGFR-positive, T790M-positive disease) bavituximab (Peregrine Pharmaceuticals) non–small cell lung cancer (previously treated advanced/metastatic disease) phase 3 belinostat (Beleodaq, Spectrum -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Assessment for Clinical Trial Eligibility Testing in a Molecular Registry (PRAEGNANT) in Germany Hanna Huebner1†, Christian M
Huebner et al. BMC Cancer (2020) 20:1091 https://doi.org/10.1186/s12885-020-07546-1 RESEARCH ARTICLE Open Access Heregulin (HRG) assessment for clinical trial eligibility testing in a molecular registry (PRAEGNANT) in Germany Hanna Huebner1†, Christian M. Kurbacher2†, Geoffrey Kuesters3, Andreas D. Hartkopf4, Michael P. Lux5, Jens Huober6, Bernhard Volz7, Florin-Andrei Taran8, Friedrich Overkamp9, Hans Tesch10, Lothar Häberle1,11, Diana Lüftner12, Markus Wallwiener13, Volkmar Müller14, Matthias W. Beckmann1, Erik Belleville15, Matthias Ruebner1, Michael Untch16, Peter A. Fasching1* , Wolfgang Janni6, Tanja N. Fehm17, Hans-Christian Kolberg18, Diethelm Wallwiener4, Sara Y. Brucker4, Andreas Schneeweiss19 and Johannes Ettl20 Abstract Background: Eligibility criteria are a critical part of clinical trials, as they define the patient population under investigation. Besides certain patient characteristics, clinical trials often include biomarker testing for eligibility. However, patient-identification mostly relies on the trial site itself and is often a time-consuming procedure, which could result in missing out on potentially eligible patients. Pre-selection of those patients using a registry could facilitate the process of eligibility testing and increase the number of identified patients. One aim with the PRAEGN ANT registry (NCT02338167) is to identify patients for therapies based on clinical and molecular data. Here, we report eligibility testing for the SHERBOC trial using the German PRAEGNANT registry. Methods: Heregulin (HRG) has been reported to identify patients with better responses to therapy with the anti- HER3 monoclonal antibody seribantumab (MM-121). The SHERBOC trial investigated adding seribantumab (MM-121) to standard therapy in patients with advanced HER2-negative, hormone receptor–positive (HR-positive) breast cancer and HRG overexpression. -

Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy
cancers Review Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy Laura Boyero 1 , Amparo Sánchez-Gastaldo 2, Miriam Alonso 2, 1 1,2,3, , 1,2, , José Francisco Noguera-Uclés , Sonia Molina-Pinelo * y and Reyes Bernabé-Caro * y 1 Institute of Biomedicine of Seville (IBiS) (HUVR, CSIC, Universidad de Sevilla), 41013 Seville, Spain; [email protected] (L.B.); [email protected] (J.F.N.-U.) 2 Medical Oncology Department, Hospital Universitario Virgen del Rocio, 41013 Seville, Spain; [email protected] (A.S.-G.); [email protected] (M.A.) 3 Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), 28029 Madrid, Spain * Correspondence: [email protected] (S.M.-P.); [email protected] (R.B.-C.) These authors contributed equally to this work. y Received: 16 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Immuno-oncology has redefined the treatment of lung cancer, with the ultimate goal being the reactivation of the anti-tumor immune response. This has led to the development of several therapeutic strategies focused in this direction. However, a high percentage of lung cancer patients do not respond to these therapies or their responses are transient. Here, we summarized the impact of immunotherapy on lung cancer patients in the latest clinical trials conducted on this disease. As well as the mechanisms of primary and acquired resistance to immunotherapy in this disease. Abstract: After several decades without maintained responses or long-term survival of patients with lung cancer, novel therapies have emerged as a hopeful milestone in this research field. -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Advanced Development of Erbb Family-Targeted Therapies in Osteosarcoma Treatment
Investigational New Drugs (2019) 37:175–183 https://doi.org/10.1007/s10637-018-0684-8 REVIEW Advanced development of ErbB family-targeted therapies in osteosarcoma treatment Wei Wang1 & Hua-fu Zhao2 & Teng-fei Yao1 & Hao Gong1 Received: 19 August 2018 /Accepted: 16 October 2018 /Published online: 24 October 2018 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Summary Osteosarcoma (OS) is the most common primary aggressive and malignant bone tumor. Newly diagnostic OS patients benefit from the standard therapy including surgical resection plus radiotherapy and neoadjuvant chemotherapy (MAP chemotherapy: high-dose methotrexate, doxorubicin and cisplatin). However, tumor recurrence and metastasis give rise to a sharp decline of the 5-year overall survival rate in OS patients. Little improvement has been made for decades, urging the development of more effective therapeutic approaches. ErbB receptor family including EGFR, HER2, HER3 and HER4, being important to the activation of PI3K/Akt and MAPK signaling pathways, are potential targets for OS treatment. Genetic aberrations (amplification, overexpression, mutation and altered splicing) of ErbB are essential to the growth, apoptosis, motility and metastasis in a variety of cancers. Overexpression of ErbB family is associated with the poor prognosis of cancer patients. A number of monoclonal antibodies or inhibitors specific for ErbB family have entered clinical trials in a range of solid tumors including breast carcinoma, lung carcinoma and sarcoma. Here, we summarized -

And Emerging, Pivotal Signalling Pathways in Metastatic Breast Cancer
REVIEW British Journal of Cancer (2017) 116, 10–20 | doi: 10.1038/bjc.2016.405 Keywords: breast cancer; aromatase inhibitor; mTOR inhibitor; PI3K inhibitor; CDK4/6 inhibitor; anastrozole; letrozole; exemestane Cotargeting of CYP-19 (aromatase) and emerging, pivotal signalling pathways in metastatic breast cancer Stine Daldorff1, Randi Margit Ruud Mathiesen1, Olav Erich Yri1, Hilde Presterud Ødegård1 and Ju¨ rgen Geisler*,1,2 1Department of Oncology, Akershus University Hospital (AHUS), Lørenskog N-1478, Norway and 2Institute of Clinical Medicine, University of Oslo, Campus AHUS, Oslo N-0313, Norway Aromatase inhibition is one of the cornerstones of modern endocrine therapy of oestrogen receptor-positive (ER þ ) metastatic breast cancer (MBC). The nonsteroidal aromatase inhibitors anastrozole and letrozole, as well as the steroidal aromatase inactivator exemestane, are the preferred drugs and established worldwide in all clinical phases of the disease. However, although many patients suffering from MBC experience an initial stabilisation of their metastatic burden, drug resistance and disease progression occur frequently, following in general only a few months on treatment. Extensive translational research during the past two decades has elucidated the major pathways contributing to endocrine resistance and paved the way for clinical studies investigating the efficacy of novel drug combinations involving aromatase inhibitors and emerging drugable targets like mTOR, PI3K and CDK4/6. The present review summarises the basic research that -

10D1F, an Anti-HER3 Antibody That Uniquely Blocks the Receptor
Published OnlineFirst January 7, 2020; DOI: 10.1158/1535-7163.MCT-19-0515 MOLECULAR CANCER THERAPEUTICS | LARGE MOLECULE THERAPEUTICS 10D1F, an Anti-HER3 Antibody that Uniquely Blocks the Receptor Heterodimerization Interface, Potently Inhibits Tumor Growth Across a Broad Panel of Tumor Models Dipti Thakkar1, Vicente Sancenon1, Marvin M. Taguiam1, Siyu Guan1, Zhihao Wu1, Eric Ng1, Konrad H. Paszkiewicz2, Piers J. Ingram1,2, and Jerome D. Boyd-Kirkup1,2 ABSTRACT ◥ In recent years, HER3 has increasingly been implicated in the activation of the PI3K pathway in a broad panel of tumor models. progression of a variety of tumor types and in acquired resistance to Even as a monotherapy, 10D1F shows superior inhibition of EGFR and HER2 therapies. Whereas EGFR and HER2 primarily tumor growth in the same cell lines both in vitro and in mouse signal through the MAPK pathway, HER3, as a heterodimer with xenograft experiments, when compared with other classes of EGFR or HER2, potently activates the PI3K pathway. Despite its anti-HER3 antibodies. This includes models demonstrating critical role, previous attempts to target HER3 with neutralizing ligand-independent activation of heterodimerization as well as antibodies have shown disappointing efficacy in the clinic, most constitutively activating mutations in the MAPK pathway. Posses- likely due to suboptimal and indirect mechanisms of action that fail sing favorable pharmacokinetic and toxicologic profiles, 10D1F to completely block heterodimerization; for example, tumors can uniquely represents a new class of anti-HER3 neutralizing anti- escape inhibition of ligand binding by upregulating ligand- bodies with a novel mechanism of action that offers significant independent mechanisms of HER3 activation. -

MM-121-02-02-10 Protocol V1.2
SHERBOC: A Double-blind, Placebo-controlled, Phase 2 trial of Seribantumab Plus Fulvestrant in Postmenopausal Women with Hormone Receptor-positive, Heregulin Positive (HRG+), HER2 Negative Metastatic Breast Cancer Whose Disease Progressed After Prior Systemic Therapy Merrimack Pharmaceuticals, Inc. Version 1.2: 28 April 2017 IND Number: 123522 EudraCT# 2017-000565-76 Sponsor: One Kendall Square Suite B7201 Cambridge, MA 02139 Confidentiality Statement This document and the information it contains is confidential and the proprietary property of Merrimack Pharmaceuticals. The information is not to be disclosed or transmitted to any party without the express approval of Merrimack Pharmaceuticals, or its agents, and any such unauthorized use or disclosure is expressly prohibited. Protocol MM-121-02-02-10 Version 1.2 – 28Apr2017 Table of Contents List of Figures ................................................................................................................................. 5 List of Tables .................................................................................................................................. 5 Abbreviations .................................................................................................................................. 6 Study Synopsis ................................................................................................................................ 9 Study Schema............................................................................................................................... -

(INN) for Biological and Biotechnological Substances
INN Working Document 05.179 Update 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 2013 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) International Nonproprietary Names (INN) Programme Technologies Standards and Norms (TSN) Regulation of Medicines and other Health Technologies (RHT) Essential Medicines and Health Products (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) © World Health Organization 2013 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int ) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected] ). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form/en/index.html ). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -
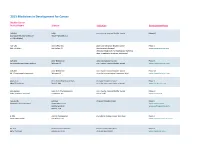
Adis R&D Insight
2015 Medicines in Development for Cancer Bladder Cancer Product Name Sponsor Indication Development Phase ABI-009 AADi non-muscle invasive bladder cancer Phase I/II (nanoparticle albumin-bound Pacific Palisades, CA mTOR inhibitor) ACP-196 Acerta Pharma platinum-refractory bladder cancer Phase II (Btk inhibitor) San Carlos, CA (combination therapy) www.acerta-pharma.com (see also head/neck, hematological, leukemia, lung, lymphoma, myeloma, pancreatic) ALT-801 Altor BioScience advanced bladder cancer, Phase II (immunotherapy fusion protein) Miramar, FL non-muscle invasive bladder cancer www.altorbioscience.com ALT-803 Altor BioScience non-muscle invasive bladder cancer Phase I/II (IL-15 superagonist complex) Miramar, FL (see also hematological, myeloma, skin) www.altorbioscience.com apatorsen OncoGenex Pharmaceuticals metastatic bladder cancer Phase II (Hsp27 inhibitor) Bothell, WA (see also lung, pancreatic, prostate) www.oncogenex.com apaziquone Spectrum Pharmaceuticals non-muscle invasive bladder cancer Phase III (DNA synthesis inhibitor) Henderson, NV (Fast Track) www.sppirx.com ASG-15ME Agensys relapsed bladder cancer Phase I (antibody drug conjugate) Santa Monica, CA www.agensys.com Seattle Genetics www.seattlegenetics.com Bothell, WA B-701 BioClin Therapeutics metastatic bladder cancer (2nd-line) Phase II (anti-FGFR3 mAb) San Ramon, CA www.bioclintherapeutics.com BC-819 BioCancell Therapeutics bladder cancer (2nd-line) Phase II (gene therapy) Jerusalem, Israel (see also pancreatic) www.biocancell.com Bladder Cancer Product Name Sponsor