Victrelis, INN-Boceprevir
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Ritonavir-Boosted HIV Protease Inhibitors
16 February 2012 EMA/CHMP/117973/2012 EMEA/H/C/002332/II/0004 Questions and answers on drug interactions between Victrelis (boceprevir) and ritonavir-boosted HIV protease inhibitors The European Medicines Agency has recommended changes to the prescribing information for Victrelis (boceprevir), a medicine used to treat hepatitis C, after a drug interaction study identified interactions between Victrelis and medicines used to treat HIV called ritonavir-boosted HIV protease inhibitors. These interactions could potentially reduce the effectiveness of these medicines if used together in patients being treated for both hepatitis C and HIV. The Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the changes to ensure doctors are informed of these interactions while further data are awaited to assess the clinical impact of these drug interaction findings on these patients. What is Victrelis? Victrelis is a medicine used to treat long-term hepatitis C genotype 1 (a disease of the liver due to infection with the hepatitis C virus) in adults with compensated liver disease who have not been treated before or whose previous treatment has failed. Compensated liver disease is when the liver is damaged but is still able to work normally. Victrelis is given in combination with two other medicines, peginterferon alfa and ribavirin. The active substance in Victrelis, boceprevir, is a protease inhibitor which blocks an enzyme called HCV NS3 protease found on the hepatitis C genotype 1 virus. Victrelis was authorised in the EU in July 2011. What is the issue with Victrelis? In January 2012, the EMA was informed of the results of a study in healthy volunteers which identified drug interactions between Victrelis and the antiviral medicines atazanavir, darunavir and lopinavir, which are used to treat HIV. -

Boceprevir (Victrelis™)
Boceprevir (Victrelis™) UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: Protease Inhibitors BRAND (generic) NAMES: Victrelis (boceprevir); 200mg strength capsule FDA-APPROVED INDICATIONS Victrelis (boceprevir) is a hepatitis C virus (HCV) NS3/4A protease inhibitor indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adult patients (18 years of age or older) with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders, partial responders, and relapsers. Victrelis must not be used as monotherapy and should only be used in combination with peginterferon alfa and ribavirin. The efficacy of Victrelis has not been studies in patients who have previously failed therapy with a treatment regimen that includes Victrelis or other HCV NS3/4A protease inhibitors. COVERAGE AUTHORIZATION CRITERIA INITIAL THERAPY Boceprevir (Victrelis) may be eligible for coverage when the following criteria are met: 1. The patient is 18 years of age or older; AND 2. The patient has a diagnosis of chronic hepatitis C (CHC) infection with confirmed genotype 1; AND 3. The patient: Has F2 or higher on the IASL, Batts-Ludwig, or Metavir fibrosis staging scales (medical record documentation required); OR Has F3 or higher on the Ishak fibrosis staging scale (medical record documentation required); OR Has cirrhosis secondary to CHC [Metavir F4, Ishak F5-6, or radiographic evidence of portal hypertension, esophageal varices, ascites (medical record documentation required)]; AND 4. The patient has contraindications to a sofosbuvir-based regimen (i.e., Sovaldi or Harvoni), in addition to Viekira Pak. -

Caracterización Molecular Del Perfil De Resistencias Del Virus De La
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Programa de doctorado en Medicina Departamento de Medicina Facultad de Medicina Universidad Autónoma de Barcelona TESIS DOCTORAL Caracterización molecular del perfil de resistencias del virus de la hepatitis C después del fallo terapéutico a antivirales de acción directa mediante secuenciación masiva Tesis para optar al grado de doctor de Qian Chen Directores de la Tesis Dr. Josep Quer Sivila Dra. Celia Perales Viejo Dr. Josep Gregori i Font Laboratorio de Enfermedades Hepáticas - Hepatitis Víricas Vall d’Hebron Institut de Recerca (VHIR) Barcelona, 2018 ABREVIACIONES Abreviaciones ADN: Ácido desoxirribonucleico AK: Adenosina quinasa ALT: Alanina aminotransferasa ARN: Ácido ribonucleico ASV: Asunaprevir BOC: Boceprevir CCD: Charge Coupled Device CLDN1: Claudina-1 CHC: Carcinoma hepatocelular DAA: Antiviral de acción directa DC-SIGN: Dendritic cell-specific ICAM-3 grabbing non-integrin DCV: Daclatasvir DSV: Dasabuvir -
PASS Information
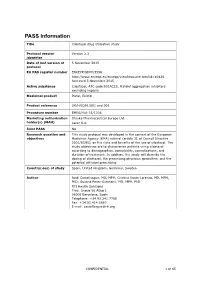
PASS Information Title Cilostazol drug utilisation study Protocol version Version 2.3 identifier Date of last version of 5 November 2015 protocol EU PAS register number ENCEPP/SDPP/3596 http://www.encepp.eu/encepp/viewResource.htm?id=10426 Accessed 5 November 2015 Active substance Cilostazol, ATC code B01AC23, Platelet aggregation inhibitors excluding heparin Medicinal product Pletal, Ekistol Product reference UK/H/0291/001 and 002 Procedure number EMEA/H/A-31/1306 Marketing authorisation Otsuka Pharmaceutical Europe Ltd. holder(s) (MAH) Lacer S.A. Joint PASS No Research question and This study protocol was developed in the context of the European objectives Medicines Agency (EMA) referral (article 31 of Council Directive 2001/83/EC) on the risks and benefits of the use of cilostazol. The study objectives are to characterise patients using cilostazol according to demographics, comorbidity, comedications, and duration of treatment. In addition, the study will describe the dosing of cilostazol, the prescribing physician specialties, and the potential off-label prescribing. Country(-ies) of study Spain, United Kingdom, Germany, Sweden Author Jordi Castellsague, MD, MPH; Cristina Varas-Lorenzo, MD, MPH, PhD; Susana Perez-Gutthann, MD, MPH, PhD RTI Health Solutions Trav. Gracia 56 Atico 1 08006 Barcelona, Spain Telephone: +34.93.241.7766 Fax: +34.93.414.2610 E-mail: [email protected] CONFIDENTIAL 1 of 65 Marketing Authorisation Holder(s) Marketing authorisation Otsuka Pharmaceutical Europe Ltd. holder(s) Gallions Wexham Springs Framewood Road Wexham SL3 6PJ, UK Lacer S.A. Sardenya 350 08025 Barcelona Spain MAH contact person Dr Marco Avila Regional Vice President, Medical Europe Otsuka Pharmaceutical Europe Ltd EU QPPV Dr Achint Kumar Otsuka Europe Development and Commercialisation Limited (OEDC) Head of Medical Dr Sanjay Kapoor Compliance-Europe Otsuka Pharmaceutical Europe Ltd. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Pegasys®) Peginterferon Alfa-2B (Peg-Intron®
Peginterferon alfa-2a (Pegasys®) Peginterferon alfa-2b (Peg-Intron®) UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: Pegylated Interferons BRAND (generic) NAME: Pegasys (peginterferon alfa-2a) Single-use vial 180 mcg/1.0 mL; Prefilled syringe 180 mcg/0.5 mL Peg-Intron (peginterferon alfa-2b) Single-use vial (with 1.25 mL diluent) and REDIPEN®: 50 mcg, 80 mcg, 120 mcg, 150 mcg per 0.5 mL. FDA-APPROVED INDICATIONS PEG-Intron (peginterferon alfa-2b): Combination therapy with ribavirin: • Chronic Hepatitis C (CHC) in patients 3 years of age and older with compensated liver disease. • Patients with the following characteristics are less likely to benefit from re-treatment after failing a course of therapy: previous nonresponse, previous pegylated interferon treatment, significant bridging fibrosis or cirrhosis, and genotype 1 infection. Monotherapy: CHC in patients (18 years of age and older) with compensated liver disease previously untreated with interferon alpha. Pegasys (Peginterferon alfa-2a): • Treatment of Chronic Hepatitis C (CHC) in adults with compensated liver disease not previously treated with interferon alpha, in patients with histological evidence of cirrhosis and compensated liver disease, and in adults with CHC/HIV coinfection and CD4 count > 100 cells/mm3. o Combination therapy with ribavirin is recommended unless patient has contraindication to or significant intolerance to ribavirin. Pegasys monotherapy is indicated for: • Treatment of adult patients with HBeAg positive and HBeAg negative chronic hepatitis B who have compensated -

PASS Information Title Cilostazol Drug Utilisation Study
PASS Information Title Cilostazol drug utilisation study Protocol version Version 2 identifier Date of last version of February 28, 2013 protocol EU PAS register number Registration is planned prior to study initiation once the protocol is final and has received regulatory endorsement by the Committee for Medicinal Products for Human Use (CHMP) Active substance Cilostazol, ATC code B01AC23, Platelet aggregation inhibitors excluding heparin Medicinal product Pletal, Ekistol Product reference UK/H/0291/001 and 002 Procedure number EMEA/H/A-31/1306 Marketing authorisation Otsuka Pharmaceutical Europe Ltd. holder(s) (MAH) Lacer S.A. Joint PASS No Research question and This study protocol was developed in the context of the European objectives Medicines Agency (EMA) referral (article 31 of Council Directive 2001/83/EC) on the risks and benefits of the use of cilostazol. The study objectives are to characterise patients using cilostazol according to demographics, comorbidity, comedications, and duration of treatment. In addition, the study will describe the dosing of cilostazol, the prescribing physician specialties, and the potential off-label prescribing. Country(-ies) of study Spain, United Kingdom, Germany, Sweden Author Jordi Castellsague, MD, MPH; Cristina Varas-Lorenzo, MD, MPH, PhD; Susana Perez-Gutthann, MD, MPH, PhD RTI Health Solutions Trav. Gracia 56 Atico 1 08006 Barcelona, Spain Telephone: +34.93.241.7766 Fax: +34.93.414.2610 E-mail: [email protected] CONFIDENTIAL 1 of 56 Marketing Authorisation Holder(s) Marketing authorisation -

Boceprevir (Victrelis)
© Hepatitis C Online PDF created September 27, 2021, 1:52 am Boceprevir (Victrelis) Discontinued. This treatment has been discontinued. Table of Contents Boceprevir Victrelis Summary Drug Summary Adverse Effects Class and Mechanism Manufacturer for United States FDA Status Indications Dosing Clinical Use Cost and Medication Access Resistance Key Drug Interactions Full Prescribing Information Figures Drug Summary Boceprevir is a first-generation hepatitis C protease inhibitor that played a valuable role in treatment of patients with genotype 1 infection during the years 2011, 2012, and 2013. With the availability of multiple, new direct-acting antiviral agents that are far superior to boceprevir, Merck has made the decision to discontinue the manufacturing of boceprevir. The relevance of boceprevir is now historical, but prior treatment failure with a boceprevir-based regimen may have resulted in development of resistance- associated variants. Adverse Effects The most common adverse effects reported with boceprevir are anemia, decreased neutrophil count, dysgeusia (alteration in taste), and vomiting. Rare cases of severe hypersensitivity reaction have been reported in patients taking boceprevir in combination with peginterferon and ribavirin. Boceprevir is clasified as pregnancy category B. Page 1/5 Class and Mechanism Boceprevir (Victrelis) is a NS3/4A protease inhibitor. Specifically, boceprevir inhibits the proteolytic cleavage of the HCV encoded polyprotein, an essential step in the viral life cycle for the production of mature forms of the viral proteins NS4A, NS4B, NS5A, and NS5B. Manufacturer for United States Boceprevir (Victrelis) is no longer manufactured in the United States. Boceprevir (Victrelis) (Figure 1) was previously manufactured by Merck & Co. Boceprevir was developed at Schering-Plough, which merged with Merck & Co. -

Inhibitor Development Against P7 Channel in Hepatitis C Virus
molecules Article Inhibitor Development against p7 Channel in Hepatitis C Virus Shukun Wei 1,2,†, Xiaoyou Hu 2,3,†, Lingyu Du 1, Linlin Zhao 1,4, Hongjuan Xue 5, Chaolun Liu 6, James J. Chou 4, Jin Zhong 2,3,6, Yimin Tong 3,*, Shuqing Wang 7,* and Bo OuYang 1,2,* 1 State Key Laboratory of Molecular Biology, Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 333 Haike Road, Shanghai 201203, China; [email protected] (S.W.); [email protected] (L.D.); [email protected] (L.Z.) 2 University of Chinese Academy of Sciences, Beijing 100049, China; [email protected] (X.H.); [email protected] (J.Z.) 3 CAS Key Laboratory of Molecular Virology and Immunology, Institute Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai 200031, China 4 Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA; [email protected] 5 National Facility for Protein Science in Shanghai, ZhangJiang Lab, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China; [email protected] 6 ShanghaiTech University, Shanghai 201210, China; [email protected] 7 School of Pharmacy, Tianjin Medical University, Tianjin 300070, China * Correspondence: [email protected] (Y.T.); [email protected] (S.W.); [email protected] (B.O.) † This authors contributed equally. Abstract: Hepatitis C Virus (HCV) is the key cause of chronic and severe liver diseases. The recent direct-acting antiviral agents have shown the clinical success on HCV-related diseases, but the rapid HCV mutations of the virus highlight the sustaining necessity to develop new drugs. -

Symmetrel (Amantadine)
An#virals Lecture 20 Biology W3310/4310 Virology Spring 2015 You can’t go back and you can’t stand s0ll. If the thunder don’t get you, then the lightning will. JERRY GARCIA The Wheel (lyrics by Robert Hunter) Vaccines can prevent viral disease • But they Have modest or no therapeuMc effect if an individual is already infected (excepMon?) • Our second arm of anMviral defense is anMvirals • Can stop infecMon once it Has started Despite 50 years of research, our arsenal of an#viral drugs remains dangerously small Only about 100 an0viral drugs are available on the US market Most against HIV, HCV, herpesviruses - Persistent infecons Example of approved Target Virus compound Attachment HIV Maraviroc Uncoating Influenza A Amantadine, rimantadine Acyclovir, valacyclovir, DNA polymerase Herpesviruses cidofovir Reverse transcriptase HIV Zidovudine, nevirapine HIV Saquinavir, ritonavir Viral protease HCV Telaprevir, boceprevir Neuraminidase Influenza A and B Zanamavir, oseltamivir Why are there so few anviral drugs? • Compounds interfering with virus growth can adversely affect the Host cell - Side effects are common (unacceptable) - Every step in viral life cycle engages host funcons • Some medically important viruses can’t be propagated, Have no animal model, or are dangerous - HBV, HPV, norovirus - Smallpox - Ebola, Lassa An unappreciated third reason may be the most important • A compound must block virus replicaMon completely! It must be potent • Many standard pHarmaceuMcals can be effecMve if enZyme acMvity is parMally blocked • ParMal inHibiMon -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

Telaprevir, Boceprevir Improved HCV Cure Rates
DECEMBER 2010 • WWW.FAMILYPRACTICENEWS.COM INFECTIOUS DISEASES 29 Telaprevir, Boceprevir Improved HCV Cure Rates BY DIANA MAHONEY arm with undetectable HCV underwent a similar 4-week standard Major Finding: The HCV protease inhibitors RNA at study weeks 8 and 12 therapy lead-in strategy as defined above, FROM THE ANNUAL MEETING OF THE telaprevir and boceprevir improve sustained followed by the addition of placebo for AMERICAN ASSOCIATION FOR virologic response rates and shorten treat- was 36 weeks, whereas those THE STUDY OF LIVER DISEASES ment duration in patients with chronic he- patients in whom HCV RNA 44 more weeks or by the addition of bo- VITALS patitis C virus infection. was detectable at study week 8, ceprevir, either for 24 more weeks for pa- tients with undetectable HCV RNA at BOSTON – The forthcoming availabili- Data Source: Four phase III clinical trials but undetectable at study week ty of the protease inhibitors telaprevir and evaluating the safety and efficacy of the di- 12, stopped boceprevir at week week 8 or for 24 more weeks plus 20 ad- boceprevir for the treatment of chronic rect-acting antivirals in treatment-naive and 36 but continued standard ther- ditional weeks of standard therapy for hepatitis C is likely to vastly improve vi- treatment-resistant HCV patients. apy for an additional 12 weeks, patients with detectable HCV RNA at rologic response rates and cut treatment Disclosures: Dr. Jacobson and Dr. Sherman for a total treatment duration week 8, but not at week 24, Dr. Poordad times, but experts warn that such ad- disclosed relationships with Vertex Pharma- of 48 weeks, Dr.