Hymenoptera Abundance on Candidate Plants for Conservation Biological Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10 June 1954 CORRIGENDA to the CONSOLIDATED SCHEDULES 1. There Is Herewith Circulated to Each Contracting Party a Draft of the C
10 June 1954 CORRIGENDA TO THE CONSOLIDATED SCHEDULES 1. There is herewith circulated to each contracting party a draft of the corrections to be made to the Consolidated Schedules in order to keep them abreast of the legal texts annexed to the General Agreement. 2. It has not been thought necessary to prepare a new page for every change. This procedure would have entailed the risk of introducing further errors. In the case of the Schedule of the Belgian Congo and Ruanda Urundi (Schedule II, Section B), however, where the whole Section has been modified, a new Section has been prepared to replace the original. 3. Accordingly, there has been prepared for each consolidated schedule which requires amendment - as listed overleaf - (i) the items which have been modified or which require substantial amendment and the new items which are to be inserted, (the texts reproduced are to replace the whole of the corresponding items or sub-items in the Consolidated Schedules, unless otherwise indicated), and (ii) a list of alterations, such as changes in punctuation marks or item numbers, deletions of items, changes of single words, etc., which can be made more quickly and conveniently by hand. 4. For changes which arise out of rectifications or modifications contained in protocols, documents, etc., the source can be conveniently traced through the "List of Changes effected by Protocols and Decisions of the CONTRACTING PARTIES" (G/75). 5. Contracting parties are requested to examine the draft and to report any additions, corrections, etc., by 10 July 1954. By that date all contracting parties should indicate the number of photo-offset copies re^ulred-.- M3T/11/54 TABLE OF CONTENTS iÈSS. -

21 CFR Ch. I (4–1–10 Edition) § 582.20
§ 582.20 21 CFR Ch. I (4–1–10 Edition) Common name Botanical name of plant source Marjoram, sweet .......................................................................... Majorana hortensis Moench. Mustard, black or brown .............................................................. Brassica nigra (L.) Koch. Mustard, brown ............................................................................ Brassica juncea (L.) Coss. Mustard, white or yellow .............................................................. Brassica hirta Moench. Nutmeg ........................................................................................ Myristica fragrans Houtt. Oregano (oreganum, Mexican oregano, Mexican sage, origan) Lippia spp. Paprika ......................................................................................... Capsicum annuum L. Parsley ......................................................................................... Petroselinum crispum (Mill.) Mansf. Pepper, black ............................................................................... Piper nigrum L. Pepper, cayenne ......................................................................... Capsicum frutescens L. or Capsicum annuum L. Pepper, red .................................................................................. Do. Pepper, white ............................................................................... Piper nigrum L. Peppermint .................................................................................. Mentha piperita L. Poppy seed -
Bay Leaves - Product Specification
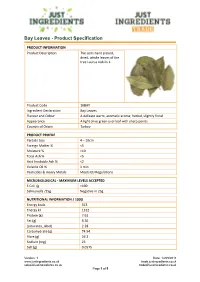
Bay Leaves - Product Specification PRODUCT INFORMATION Product Description The semi hand picked, dried, whole leaves of the tree Laurus nobilis L. Product Code 10BAY Ingredient Declaration Bay Leaves Flavour and Odour A delicate warm, aromatic aroma; herbal, slightly floral Appearance A light olive green oval leaf with sharp points Country of Origin Turkey PRODUCT PROFILE Particle Size 4 – 10cm Foreign Matter % <5 Moisture % <10 Total Ash % <5 Acid Insoluble Ash % <2 Volatile Oil % 1 min Pesticides & Heavy Metals Meets EU Regulations MICROBIOLOGICAL - MAXIMUM LEVELS ACCEPTED E Coli /g <100 Salmonella /25g Negative in 25g NUTRITIONAL INFORMATION / 100G Energy kcals 313 Energy kJ 1312 Protein (g) 7.61 Fat (g) 8.36 {saturates_label} 2.28 Carbohydrate (g) 74.94 Fibre (g) 26.3 Sodium (mg) 23 Salt (g) 0.0575 Version: 1 Date: 14/05/2018 www.justingredients.co.uk trade.justingredients.co.uk [email protected] [email protected] Page 1 of 5 Bay Leaves - Product Specification INTOLERANCE AND ALLERGEN INFORMATION Please ensure you have read our Allergen Policy statement available here. For further information about allergen handling by JustIngredients and its suppliers, please read our online guide here. Key: ✓ Indicates where a product is an allergen or where an allergen has intentionally been added to the final product. Cereal/Wheat products Nut and nut products Peanuts and products thereof Soybean and products thereof Sesame seed and products thereof Mustard and products thereof Milk and Dairy Products Products containing Sulphur -

Cytotoxic Activity of Essential Oils from Labiatae and Lauraceae Families Against in Vitro Human Tumor Models
ANTICANCER RESEARCH 27: 3293-3300 (2007) Cytotoxic Activity of Essential Oils from Labiatae and Lauraceae Families Against In Vitro Human Tumor Models MONICA ROSA LOIZZO1, ROSA TUNDIS1, FEDERICA MENICHINI1, ANTOINE MIKAEL SAAB2, GIANCARLO ANTONIO STATTI1 and FRANCESCO MENICHINI1 1Faculty of Pharmacy, Nutrition and Health Sciences, Department of Pharmaceutical Sciences, University of Calabria, I-87036 Rende (CS), Italy; 2Faculty of Sciences II, Chemistry Department, Lebanese University, P.O. Box :90656 Fanar, Beirut, Lebanon Abstract. Background: The aim of this work was to study undertaken on the cytotoxic activity of essential oils from the cytotoxicity of essential oils and their identified Sideritis perfoliata, Satureia thymbra, Salvia officinalis, Laurus constituents from Sideritis perfoliata, Satureia thymbra, nobilis or Pistacia palestina. Salvia officinalis, Laurus nobilis and Pistacia palestina. The genus Sideritis (Labiatae) is of great botanical and Materials and Methods: Essential oils were obtained by pharmacological interest, in fact many species are reported hydrodistillation and were analysed by gas chromatography to have analgesic, anti-inflammatory, antibacterial, (GC) and GC/mass spectrometry (MS). The cytotoxic activity antirheumatic, anti-ulcer, digestive and vaso-protective was evaluated in amelanotic melanoma C32, renal cell properties and have been used in Mediterranean folk adenocarcinoma ACHN, hormone-dependent prostate medicine (11). No reports have been found concerning the carcinoma LNCaP, and MCF-7 breast cancer cell lines by phytochemical composition or biological or cytotoxic activity the sulforhodamine B (SRB) assay. Results: L. nobilis fruit of S. perfoliata (12). S. thymbra (Labiatae) is the most oil exerted the highest activity with IC50 values on C32 and common Satureja specimen and is known as a herbal home ACHN of 75.45 and 78.24 Ìg/ml, respectively. -

21 CFR Ch. I (4–1–17 Edition) § 182.20
§ 182.20 21 CFR Ch. I (4–1–17 Edition) Common name Botanical name of plant source Marigold, pot ........................................................... Calendula officinalis L. Marjoram, pot .......................................................... Majorana onites (L.) Benth. Marjoram, sweet ...................................................... Majorana hortensis Moench. Mustard, black or brown ......................................... Brassica nigra (L.) Koch. Mustard, brown ....................................................... Brassica juncea (L.) Coss. Mustard, white or yellow ......................................... Brassica hirta Moench. Nutmeg .................................................................... Myristica fragrans Houtt. Oregano (oreganum, Mexican oregano, Mexican Lippia spp. sage, origan). Paprika .................................................................... Capsicum annuum L. Parsley .................................................................... Petroselinum crispum (Mill.) Mansf. Pepper, black .......................................................... Piper nigrum L. Pepper, cayenne ..................................................... Capsicum frutescens L. or Capsicum annuum L. Pepper, red ............................................................. Do. Pepper, white .......................................................... Piper nigrum L. Peppermint .............................................................. Mentha piperita L. Poppy seed ............................................................ -

Iskogen #0 Yule
i Skogen! - by Eleonora Matarrese © 2017 Eleonora Matarrese and respective authors All rights reserved Number 0 - Autumn - September 21st - December 21st, 2017 Cover photo: cover of The Old Willow Tree and other stories by Carl Ewald; illustration by Helen Mary Jacobs. First publication October 1921, London, UK i Skogen! is an electronic publication because we do not want to consume paper and want to preserve trees. If we decide for a printed version it will definitely be on recycled paper and environmentally friendly sources. i Skogen! is a homemade publication without advertising for choice. The companies and people mentioned have paid nothing to authors/publisher and are quoted only for their amazing work in Italy in the wild food field. i Skogen! is a non-party, non-political publication; a publication also for children to which illustrate botany and the secrets of Nature under the supervision of an adult . i Skogen! declines any responsibility for improper use of information provided, both for food and herbal remedies and for all botanical and illustrative information on wild herbs collection. Its texts are a research work and those in the health field do not replace the opinion of a professional herbalist and physician. i Skogen! wishes to spread a full-spectrum knowledge about plants and the balance of the human being in the world around us, even through a greater awareness of the living beings that are part of the flora of our planet. Eventually, i Skogen! wants to propagate a culture that is lost, at least in Italy, and to retrieve the agricultural traditions of our European ancestors, and not only this: for this reasons, sections dedicated to folklore embrace traditions that do not cover the dogma of monotheistic religions, and excavate backwards for a healthy philological and historical recovery. -

Effect of Enzymatic, Ultrasound, and Reflux Extraction Pretreatments On
View metadata, citation and similar papers at core.ac.uk brought to you by CORE molecules Article Effect of Enzymatic, Ultrasound, and Reflux Extraction Pretreatments on the Chemical Composition of Essential Oils Andela¯ Miljanovi´c 1, Ana Bielen 1,*, Dorotea Grbin 1, Zvonimir Marijanovi´c 2 , Martina Andlar 1, TonˇciRezi´c 1 , SunˇcicaRoca 3 , Igor Jerkovi´c 2 , Dražen Viki´c-Topi´c 3,4 and Maja Dent 1,* 1 Faculty of Food Technology and Biotechnology, University of Zagreb, Pierottijeva 6, 10 000 Zagreb, Croatia; [email protected] (A.M.); [email protected] (D.G.); [email protected] (M.A.); [email protected] (T.R.) 2 Faculty of Chemistry and Technology, University of Split, Rudera¯ Boškovi´ca35, 21 000 Split, Croatia; [email protected] (Z.M.); [email protected] (I.J.) 3 NMR Centre, Ruder¯ Boškovi´cInstitute, Bijeniˇckacesta 54, 10 000 Zagreb, Croatia; [email protected] (S.R.); [email protected] (D.V.-T.) 4 Department of Natural and Health Sciences, Juraj Dobrila University of Pula, Zagrebaˇcka 30, 52 100 Pula, Croatia * Correspondence: [email protected] (A.B.); [email protected] (M.D.); Tel.: +385-98-179-3307 (A.B.); +385-91-444-0555 (M.D.) Academic Editor: Petras Rimantas Venskutonis Received: 8 September 2020; Accepted: 19 October 2020; Published: 20 October 2020 Abstract: The effect of different hydrodistillation pretreatments, namely, reflux extraction, reflux extraction with the addition of cell wall-degrading enzymes, and ultrasound, on the yield and chemical composition of essential oils of sage, bay laurel, and rosemary was examined. All pretreatments improved essential oil yield compared to no-pretreatment control (40–64% yield increase), while the oil quality remained mostly unchanged (as shown by statistical analysis of GC-MS results). -

Appendix 1 Vernacular Names
Appendix 1 Vernacular Names The vernacular names listed below have been collected from the literature. Few have phonetic spellings. Spelling is not helped by the difficulties of transcribing unwritten languages into European syllables and Roman script. Some languages have several names for the same species. Further complications arise from the various dialects and corruptions within a language, and use of names borrowed from other languages. Where the people are bilingual the person recording the name may fail to check which language it comes from. For example, in northern Sahel where Arabic is the lingua franca, the recorded names, supposedly Arabic, include a number from local languages. Sometimes the same name may be used for several species. For example, kiri is the Susu name for both Adansonia digitata and Drypetes afzelii. There is nothing unusual about such complications. For example, Grigson (1955) cites 52 English synonyms for the common dandelion (Taraxacum officinale) in the British Isles, and also mentions several examples of the same vernacular name applying to different species. Even Theophrastus in c. 300 BC complained that there were three plants called strykhnos, which were edible, soporific or hallucinogenic (Hort 1916). Languages and history are linked and it is hoped that understanding how lan- guages spread will lead to the discovery of the historical origins of some of the vernacular names for the baobab. The classification followed here is that of Gordon (2005) updated and edited by Blench (2005, personal communication). Alternative family names are shown in square brackets, dialects in parenthesis. Superscript Arabic numbers refer to references to the vernacular names; Roman numbers refer to further information in Section 4. -

How to Care for a SWEET BAY (HERB) Tree Laurus Nobilis SELECT SITE
How to Care for a SWEET BAY (HERB) Tree Laurus nobilis SELECT SITE: Light Requirements: Full Sun (more than 6 hrs/day) is ideal; Part Sun (4-6 hrs/day) is okay. A South facing window and/or location is best. Recommended use is as an attractive houseplant. This tree will grow larger, in its native Mediterranean habitat, it typically reaches 40’ or more. In pots, the tree can be easily trimmed to desired size because it is a very slow growing plant. Thrives in an organically rich, fast draining potting soil. Garden Uses: Culinary or potpourri houseplant. Ideal USDA Plant Hardiness Zones: 8,9,10,11 (must be used as a houseplant in every other Zone) PLANT PREP: Open box upon arrival, remove packing materials to let the plant “breathe”. Remove plastic bag, tape, and newspaper. Water well. There are drainage holes in the bottom of the pot so do this over a sink basin where the extra water will drain away. PLANTING/REPOTTING: Transplant the plant into a larger pot, upon arrival and in any future years, when the roots of the plant have become crowded but before becoming root bound. Choose a container that is 1-2” wider than the current pot, with a drainage hole in the bottom. Use an all-purpose potting mix (not garden soil) and begin by filling the new pot about ½ full of potting mix. Remove the plant from its pot and set the root ball on top of the mix. Adjust the height so that the top of the roots sit about 1” below the rim. -

Protec Botanica Product List
Botanica flo iva flo iva Botanically Inspired Solutions. Naturally. Essential Oils Botanical name Part of plant used Country of origin Floviva Almond Oil Bitter Prunus amygdalus dulcis Kernel USA Floviva Amyris Oil Amyris balsamifera L. Wood Haiti Floviva Angelica Oil Angelica archangelica L. Root / Seed France / Hungary Floviva Aniseed Oil Pimpinella anisum L. Seed Spain / India Floviva Anthopogon Oil Rhododendron anthopogon Leaf & twig Nepal Floviva Basil Oil Ocimum basilicum L. Aerial part Madagascar / Egypt / Vietnam / S. Asia Floviva Bay Laurel Oil Laurus nobilis L. Leaf Albania / Bosnia Herzegovina / C. Europe Floviva Bay Oil WI Pimenta recemosa Leaf Dominica / Jamaica Floviva Benzoin Resinoid Styrax tonkinensis Resin Laos / Thailand Floviva Bergamot Oil Citrus aurantium L. bergamia Peel Calabria, Italy Floviva Bergamot Oil FCF Citrus aurantium L. bergamia Peel Calabria, Italy Floviva Birch Oil Sweet Betula lenta Bark USA Floviva Black Pepper Oil Piper nigrum L. Seed India / Madagascar Floviva Black Spruce Oil Picea mariana Needle Canada Floviva Blue Tansy Oil Tanacetum annuum L. Flower Morocco Floviva Cabreuva Oil Myrocarpus fastigiatus Bark Brazil / Paraguay Floviva Cade Oil Juniperus oxycedrus Wood France / Spain Floviva Cajeput Oil Melaleuca leucadendron L. Leaf & twig Vietnam / Indonesia Floviva Camphor Oil Cinnamomum camphora L. Wood China Floviva Caraway Oil Carum carvi L. Seed Hungary / Russia Floviva Cardamon Oil Elettaria cardamomum L. Seed Guatemala / Sri Lanka / PNG Floviva Carrot Seed Oil Daucus carota L. Seed France Floviva Cassia Oil Cinnamomum cassia L. Bark / Leaf China Floviva Catnip Oil Nepeta cataria Leaf and flower France / USA Floviva Cedarwood Oil Cedrus atlantica Wood Morocco / USA / China Floviva Celery Oil Apium graveolens L. -

Quality Oriented Drying of Lemon Balm (Melissa Officinalis L.)
FORSCHUNGSBERICHT AGRARTECHNIK des Fachausschusses Forschung und Lehre der Max-Eyth-Gesellschaft Agrartechnik im VDI (VDI-MEG) 498 Sandra Patricia Cuervo-Andrade Quality oriented drying of Lemon Balm (Melissa officinalis L.) Dissertation Witzenhausen 2011 ISSN 0931-6264 Universität Kassel Fachbereich Ökologische Agrarwissenschaften Fachgebiet Agrartechnik Prof. Dr. Oliver Hensel Quality oriented drying of Lemon Balm (Melissa officinalis L.) Dissertation zur Erlangung des akademischen Grades einer Doktorin der Agrarwissenschaften (Dr. agr.) von Dipl.-Ing. Sandra Patricia Cuervo-Andrade aus Bogotá, Kolumbien 2011 Die vorliegende Arbeit wurde vom Fachbereich Ökologische Agrarwissenschaften der Universität Kassel als Dissertation zur Erlangung des akademischen Grades „Doktorin der Agrarwissenschaften“ angenommen. Tag der mündlichen Prüfung: 21.03.2011 Erster Gutachter: Prof. Dr. Oliver Hensel Zweiter Gutachter: Prof. Dr. Werner Hofacker Mündliche Prüfer: PD Dr. Jens Gebauer PD Dr. Johannes Kahl Alle Rechte vorbehalten. Die Verwendung von Texten und Bildern, auch auszugsweise, ist ohne Zustimmung des Autors urheberrechtswidrig und strafbar. Das gilt insbesondere für Vervielfältigung, Übersetzung, Mikroverfilmung sowie die Einspeicherung und Verarbeitung in elektronischen Systemen. © 2011. Im Selbstverlag: Sandra Patricia Cuervo Andrade Bezugsquelle: Universität Kassel, FB Ökologische Agrarwissenschaften Fachgebiet Agrartechnik Nordbahnhofstr. 1a 37213 Witzenhausen Acknowledgements This work was developed in the department of agricultural engineering at the University of Kassel in Witzenhausen led by Prof.Dr.Oliver Hensel. I particularly want to thank to Prof.Dr. Oliver Hensel for his continued support in carrying out the work. His technical suggestions were crucial to the successful conclusion of the work. His good sense of humor always kept me motivated to continue and overcome the difficulties inherent in the research. I also want to thank to Prof.Dr. -

Proximate and Some Phytochemical Constituents of Three West African Vegetable Spices
Eurasian Journal of Food Science and Technology 2021; Vol:5, Issue:1, pp:59-66 Proximate and Some Phytochemical Constituents of Three West African Vegetable Spices Akinola Victor L*1, Salami Adams A1, Durodola Olamide I 1,*, Olasoju Abayomi S2, Ogunsina Babatunde S1. 1Department of Agricultural and Environmental Engineering, Obafemi Awolowo University, Ile Ife, Nigeria 2Department of Agricultural Science Education, Adeniran Ogunsanya College of Education, Lagos, Nigeria. *Corresponding author: [email protected] ORCID: 0000-0002-6110-1011 Abstract The proximate and some phytochemical composition of three African vegetable spices, namely celery, bay, and scent leaves, were investigated in this study. Fresh samples of the spices were obtained from a local market. They were cleaned, sorted, dried, and blended to reduce the particle size to flour. From the results of the analysis, the particle size of the scent, bay, and celery leaves flour ranged from coarse to fine size in that order, respectively. The water absorption capacity of celery leaf flour was greater among others, but bay leaf flour's oil absorption capability was the highest. The moisture content, fat, and crude fibre content of the spices flour ranged between 10.71 –10.84%, 1.81 – 4.5%, and 4.44 – 11.41%, respectively. While the ash, protein, and carbohydrate content were 2.18 – 8.69%, 0.92 – 14.88%, and 61.28 – 71.88%, respectively. The concentration of alkaloids, phenols, tannins, and saponins was higher in scent leaf flour when compared to others. However, flavonoids were more present in celery leaf flour. The large proportion of phytochemicals contents of the spices suggest that they are a good source of minerals.