Sonicator Dyeing of Cotton, Wool and Silk with the Leaves Extract
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
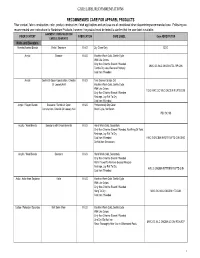
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Osher Lifelong Learning Institute
USDA-ARS National Plant Germplasm System Conservation of Fruit & Nut Genetic Resources Joseph Postman Plant Pathologist & Curator National Clonal Germplasm Repository Corvallis, Oregon May 2010 Mission: Collect – Preserve Evaluate – Enhance - Distribute World Diversity of Plant Genetic Resources for Improving the Quality and Production of Economic Crops Important to U.S. and World Agriculture Apple Accessions at Geneva Malus angustifolia ( 59 Accessions) Malus sikkimensis ( 14 Accessions) Malus baccata ( 67 Accessions) Malus sp. ( 41 Accessions) Malus bhutanica ( 117 Accessions) Malus spectabilis ( 9 Accessions) Malus brevipes ( 2 Accessions) Malus sylvestris ( 70 Accessions) Malus coronaria ( 98 Accessions) Malus toringo ( 122 Accessions) Malus domestica ( 1,389 Accessions) Malus transitoria ( 63 Accessions) Malus doumeri ( 2 Accessions) Malus trilobata ( 2 Accessions) Malus florentina ( 4 Accessions) Malus tschonoskii ( 3 Accessions) Malus floribunda ( 12 Accessions) Malus x adstringens ( 2 Accessions) Malus fusca ( 147 Accessions) Malus x arnoldiana ( 2 Accessions) Malus halliana ( 15 Accessions) Malus x asiatica ( 20 Accessions) Malus honanensis ( 4 Accessions) Malus x astracanica ( 1 Accessions) Malus hupehensis ( 185 Accessions) Malus x atrosanguinea ( 2 Accessions) Malus hybrid ( 337 Accessions) Malus x dawsoniana ( 2 Accessions) Malus ioensis ( 72 Accessions) Malus x hartwigii ( 5 Accessions) Malus kansuensis ( 45 Accessions) Malus x magdeburgensis ( 2 Accessions) Malus komarovii ( 1 Accessions) Malus x micromalus ( 25 Accessions) -

Textile Printing
TECHNICAL BULLETIN 6399 Weston Parkway, Cary, North Carolina, 27513 • Telephone (919) 678-2220 ISP 1004 TEXTILE PRINTING This report is sponsored by the Importer Support Program and written to address the technical needs of product sourcers. © 2003 Cotton Incorporated. All rights reserved; America’s Cotton Producers and Importers. INTRODUCTION The desire of adding color and design to textile materials is almost as old as mankind. Early civilizations used color and design to distinguish themselves and to set themselves apart from others. Textile printing is the most important and versatile of the techniques used to add design, color, and specialty to textile fabrics. It can be thought of as the coloring technique that combines art, engineering, and dyeing technology to produce textile product images that had previously only existed in the imagination of the textile designer. Textile printing can realistically be considered localized dyeing. In ancient times, man sought these designs and images mainly for clothing or apparel, but in today’s marketplace, textile printing is important for upholstery, domestics (sheets, towels, draperies), floor coverings, and numerous other uses. The exact origin of textile printing is difficult to determine. However, a number of early civilizations developed various techniques for imparting color and design to textile garments. Batik is a modern art form for developing unique dyed patterns on textile fabrics very similar to textile printing. Batik is characterized by unique patterns and color combinations as well as the appearance of fracture lines due to the cracking of the wax during the dyeing process. Batik is derived from the Japanese term, “Ambatik,” which means “dabbing,” “writing,” or “drawing.” In Egypt, records from 23-79 AD describe a hot wax technique similar to batik. -

Genetic Divergence Studies in Indigenous Malus Baccata Biotypes
International Journal of Chemical Studies 2019; 7(3): 4237-4244 College of Medicine, Hebei University, Baoding 071000, China P-ISSN: 2349–8528 E-ISSN: 2321–4902 IJCS 2019; 7(3): 4237-4244 Genetic divergence studies in indigenous Malus © 2019 IJCS Received: 16-03-2019 baccata biotypes by using the random amplified Accepted: 18-04-2019 decamer primers Vikrant Department of Biotechnology, Dr YS Parmar University of Vikrant and Manju Modgil Horticulture and Forestry, Nauni, Solan,Himachal Pradesh, Abstract India Study of genetic diversity is an important aspect to be covered for proper utilization of the germplasm for ManjuModgil breeding purpose and crop improvement programme. Keeping in view, genetic divergence studies in Department of Biotechnology, indigenous crab apple biotypes (Malus baccata var. Himaliaca) maintained at two field gene banks of Dr YS Parmar University of Himachal Pradesh state of India was carried out by using the RAPD molecular markers. A total of 119 Horticulture and Forestry, decamer primers were initially screened to check these biotypes during the genotypic screening out of Nauni, Solan, Himachal which 94 showed clear and scorable bands. In samples collected from IARI Regional Station, Shimla, Pradesh, India these primers revealed 67.47% of polymorphism and PIC value ranged between 0.497 to 0.867, whereas average number of alleles per primer was 4.84. Jaccard’s similarity coefficient ranged from 0.44 to 0.65 which showed the divergence among the biotypes. Comparatively low percentage polymorphism (53.37%) was observed in seven biotypes maintained at NBPGR, Regional Station, Shimla, while almost similar PIC value range 0.47-0.87 was obtained as in case of former. -

Finding List and Guide to Tllc Secrest Arboretum
SPECIAL CIRCULAR 91 MAY 1960 REV ISED Finding List and Guide to tllc Secrest Arboretum Wood Utilization Research Laboratory and Secrest Arboretum Headquarters Effective July 1, 1965, the name of the Ohio Agricultural Experiment Station was changed to: 0 Ohio Agricu ltura I Research and Development Center, ')ll Wooster, Ohio • This page intentionally blank. • GUIBE TO THE SECREST ARBORETUM OHIO AGRICULTURAL EXPERJMENT STATION WOOSTER, OHIO '/ By John E. Aughanbaugh, Harry R. Muckley, and Oliver D. Diller* In May 1950 the forest and ornamental plantings at the Ohio Agricultural Experiment Station were dedicated as the Secrest Arboretum in memory of Edmund Secrest, the father of forestry in Ohio. Since 19o8 these plantings have been expanded to include well over 600 species and varieties of trees and shrubs from many parts of the world. It is the purpose of this publication to serve as a finding list and guide to the Arboretum. The Purpose of the Arboretum One purpose of the Arboretum is to _determine the species and varieties of trees adapted for ornamental, windbreak, and shelterbelt uses in 6hio. There are many varieties of spruces, firs, yews, arborvitae, and other coniferous trees and shrubs growing here for observation by people interested in landscaping and the planting of shelterbelts. Among the more recent additions to the Arboretum is a collection of over 60 varieties of flowering crabapples, 57 selections of hollies and a plot of Chinese dawnredwood. The second purpose of the Arboretum is to determine the spe?ies of trees best adapted for reforestation in Ohio and to determine the silvicultural requirements that will obtain best results in growth and maturity. -

Material/Source Features Pros Cons Polyester/Cotton Blend 80% Polyester / 20% Cotton, 65/35, and 40/60 Are Common Blends. • La
Material/Source Features Pros Cons Polyester/Cotton Blend Liquid Resistance Appropriate for use in Polyester blends burn Splash resistant. clinical settings and readily when ignited, 80% Polyester / 20% No specific chemical resistance. research laboratories and are not appropriate Cotton, 65/35, and 40/60 Anecdotal evidence suggests polyester blends provide better protection against where biological for use with flammable are common blends. corrosive material than does cotton. material is liquids, pyrophoric Flame Resistance manipulated. materials, or near open No flame. Lab Supply Polyester blends burn more readily than 100% cotton or flame-resistant Companies materials. Chemistry Comfort Stockroom Lightweight and breathable. Biology More cotton in the blend results in better breathability. Stockroom 100% Cotton Liquid Resistance Appropriate for use in Cotton lab coats should Not splash resistant. clinical settings and be supplemented with a Lab Supply No specific chemical resistance. research laboratories chemical splash apron Companies Anecdotal evidence suggests cotton lab coats provide better protection from where there is light when corrosive material Chemistry solvent contamination than corrosive contamination. flammable liquid or is handled. Stockroom Flame Resistance open flame use. Biology No Stockroom Burns less readily than polyester blends. Comfort Lightweight and breathable. 100% Cotton treated with Liquid Resistance Appropriate for use in More costly than a flame retardant. Not splash resistant. research laboratories traditional 100% cotton No specific chemical resistance. where substantial fire lab coat. Lab Supply Anecdotal evidence suggests cotton lab coats provide better protection from risk exists from Companies solvent contamination than corrosive contamination. flammable material Manufacturers of Flame Resistance handling or open flame flame-resistant Yes use. -

Fabric Fiber Content
Fabric Types, Count & Fiber Content Zweigart Linen Count Content Belfast 32 100% linen Afghans - 100% Polyacrylic Cashel 28 100% linen Abby 18ct Alba 14ct Almanac 14ct Cork 19 100% linen Anne Cloth 18ct Baby Snuggle 18ct Country Home 18ct Dublin 25 100% linen Diamond 18ct Gloria 14ct Hearthside 14ct Edinborough 36 100% linen Honeycomb 18ct Novara 14ct Patrice 14ct Fine Linen 45 55% linen + 45% cotton Afghans - 100% Cotton Glasgow 28 100% linen Anne Cloth 18ct Augusta 14 ct Novara 14ct Kingston 50 100% linen Teresa 14ct Newcastle 40 100% linen Afghans- Misc Normandie 55% cotton + 45% linen Pastel LinenD 28 52% cotton + 48% linen Gloria 14ct 70% rayon + 30% linen Pearl Linen 20, 25, 28 60% polyester + 40% linen Merino 28ct 100% Wool Mosaik 18ct 52% cotton + 42% rayon Patterned Count Content Tannenbaum 18ct 52% cotton + 42% rayon Cottage Huck 14 100% cotton Aida Weave Count Content Belinda 20 52% cotton + 48% rayon Diana 20 52% cotton + 48% rayon Aida 8, 11, 14, 16, 18 100% cotton Newport 28 100% linen Country AidaD 7 100% polyacrylic Sambuca 28 60% polyester + 40% linen Damask Aida 11,14,18 52% cotton + 48% rayon Saronno 28 52% cotton + 48% rayon GoldauD 7 55% rayon + Shenandoah 28 55% linen + 45% rayon 40% cotton + 5% metallic Hardanger 22 100% cotton Canvas Count Content Hearthstone 14 60% cotton + 40% linen Congress 24 100% cotton Herta 6 100% cotton Congressa 24 100% cotton Huck 14 100% cotton Cordova 22 100% cotton Klostern 7 60% rayon + 40% cotton Double Mesh 5, 6.5, 7.5, 10, 12, Linen Hardanger 22 100% linen 14, 16, 18, 20 100% cotton -

Biodiversity in Karnali Province: Current Status and Conservation
Biodiversity in Karnali Province: Current Status and Conservation Karnali Province Government Ministry of Industry, Tourism, Forest and Environment Surkhet, Nepal Biodiversity in Karnali Province: Current Status and Conservation Karnali Province Government Ministry of Industry, Tourism, Forest and Environment Surkhet, Nepal Copyright: © 2020 Ministry of Industry, Tourism, Forest and Environment, Karnali Province Government, Surkhet, Nepal The views expressed in this publication do not necessarily reflect those of Ministry of Tourism, Forest and Environment, Karnali Province Government, Surkhet, Nepal Editors: Krishna Prasad Acharya, PhD and Prakash K. Paudel, PhD Technical Team: Achyut Tiwari, PhD, Jiban Poudel, PhD, Kiran Thapa Magar, Yogendra Poudel, Sher Bahadur Shrestha, Rajendra Basukala, Sher Bahadur Rokaya, Himalaya Saud, Niraj Shrestha, Tejendra Rawal Production Editors: Prakash Basnet and Anju Chaudhary Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Acharya, K. P., Paudel, P. K. (2020). Biodiversity in Karnali Province: Current Status and Conservation. Ministry of Industry, Tourism, Forest and Environment, Karnali Province Government, Surkhet, Nepal Cover photograph: Tibetan wild ass in Limi valley © Tashi R. Ghale Keywords: biodiversity, conservation, Karnali province, people-wildlife nexus, biodiversity profile Editors’ Note Gyau Khola Valley, Upper Humla © Geraldine Werhahn This book “Biodiversity in Karnali Province: Current Status and Conservation”, is prepared to consolidate existing knowledge about the state of biodiversity in Karnali province. The book presents interrelated dynamics of society, physical environment, flora and fauna that have implications for biodiversity conservation. -

Fabric Supplier List
FABRIC SUPPLIER LIST CANADA Kendor Textiles Ltd 1260 Cliveden Ave Delta BC V3M 6Y1 Canada 604.434.3233 [email protected] www.kendortextiles.com Fabrics Available: Fabric supplier. Eco-friendly. Organic. Knits: solids, prints, yarn dyes and warp. Wovens: solids and yarn dyes. End Use: activewear, bottomweights, medical, lingerie, childrenswear, swimwear, rainwear, skiwear and uniform. Natural & eco items include cottons, bamboo's, modals, linens, hemps, organic cottons & organic linens. Technical items include waterproof/breathable soft shells, antibacteric & wicking polyester & recycled polyesters. Is a proud representative of the British Millerain line of waxed cottons and wools, and are able to provide custom souring. Minimums: Carries stock. In-stock minimum: 5 yards/color. Minimum order for production: 10 yards/color. Gordon Fabrics LTD #1135-6900 Graybar Rd. Richmond BC Canada 604.275.2672 [email protected] Fabrics Available: Fabric Supplier. Importer. Jobber. Carries stock. Knits & Wovens: solids, prints, yarn dyes and novelties. End Use: activewear, borromweights, eveningwear/bridal, medical, lingerie and childrenswear. Minimums: In stock minimum 1 yard. Minimum order for production varies. StartUp Fashion Supplier List 2016 – Page 1 CHINA Ecopel (HX) Co., Ltd. China +86 216.767.9686 www.ecopel.cn Fabrics Available: Fake fur and leather garments. End Uses: Childrenswear, Menswear, Other, Womenswear. Minimums: Min. order 50-100 m Hangzhou New Design Source Textile Co., Ltd. China +86 057.182.530528 Fabrics Available: Knits, Polyester/Man-Made, Prints. End Uses: Juniors Fashion, Menswear, Womenswear. Minimums: Min order 50 m. Nantong Haukai Textile Co., Ltd. China +86 513.890.78626 www.huakaitex.com Fabrics Available: Cotton, Linen. End Uses: Corporatewear/Suiting, Menswear, Womenswear. -

Development of Novel Carbon Fiber Produced from Waste Fiber by Cabonization
Journal of Oleo Science Copyright ©2012 by Japan Oil Chemists’ Society J. Oleo Sci. 61, (10) 593-600 (2012) Development of Novel Carbon Fiber produced from Waste Fiber by Cabonization Naohito Kawasaki* , Hisato Tominaga, Fumihiko Ogata, Kenji Inoue and Moe Kankawa Faculty of Pharmacy, Kinki University, (3-4-1, Kowakae, Higashi-Osaka, Osaka 577-8502, JAPAN) Abstract: The volume of waste fiber has increased rapidly in recent years, and this trend is expected to continue. In this study, therefore, we attempted to convert waste fiber to carbonaceous materials by carbonization and investigated the basic properties of the resulting carbonized fibers. The results demonstrated that pores tend to form and specific surface areas change substantially, depending on the carbonization conditions. The carbonization conditions resulting in the largest specific surface areas included a temperature increase and retention times of 2 h. Carbonization temperatures resulting in the maximum values of 1000℃ were 900–1000℃ for wool and 1000℃ for both polyester and cotton. In particular, the specific surface area of cotton after carbonization at 1000℃ was 1253 m2/g, and scanning electron microscopy (SEM) micrographs showed that cotton retained its fibrous form after carbonization. Thus, it is possible to inexpensively convert waste fibers to carbonaceous material by carbonization. The results indicate that for cotton fiber in particular, the practical application of this process to the production of low-cost fibrous activated carbon would be possible, since cotton fiber retains its fibrous form under carbonization. Key words: Waste Fiber; Carbonization; Pore Size Distribution; Conversion 1 INTRODUCTION responsibility in their pursuit of profits. However, recycling In recent years, increases in population and industrial rates for fiber products have not been rising, largely activity have led to many mutually related problems world- because of consumer resistance to the use of recycled wide concerning the environment, resources, and energy. -

Eco-Friendly Dyeing of Wool and Pashmina Fabric Using Quercus Robur L. (Fruit Cups) Dye and Salix Alba L
AL SC R IEN TU C A E N F D O N U A N D D Journal of Applied and Natural Science 7 (1) : 138 – 143 (2015) A E I T L I O P JANS N P A ANSF 2008 Eco-friendly dyeing of wool and pashmina fabric using Quercus robur L. (fruit cups) dye and Salix alba L. (wood extract) mordant Syed Maqbool Geelani1*, Shoukat Ara1, Pradeep Kumar Mishra2, S.J.A. Bhat3, Syed Hanifa4, Shamsul Haq1, Iqbal Jeelani5, Gazala Qazi1, Asif H Sofi6, Shakeel A Mir5, P.A. Khan3, Sarfaraz A Wani6 and A S.M. Raja7 1Division of Environmental Sciences, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar-190025 (J&K), INDIA. 2Department of Chemical Engineering & Technology, Indian Institute of Technology (Banaras Hindu University), Varanasi-221005.(UP), INDIA 3Faculty of Forestry, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Wadura-193201, (J&K), INDIA 4Department of Food Sciences & Technology, University of Kashmir, Hazratbal, Srinagar-190006 (J&K), INDIA 5Division of Agricultural Statistics, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar-190025 (J&K), INDIA 6Division of Livestock Products Technology, Faculty of Veterinary Science & Animal Husbandry, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shuhama-190006 (J&K), INDIA 7Division of Textile Manufacture and Textile chemistry, Central Sheep and wool Research Institute, Avikanagar - 304501 (Rajasthan), INDIA *Corresponding author. E-mail: [email protected] Received: November 24, 2014; Revised received: February 20, 2015; Accepted: March 19, 2015 Abstract: Study was conducted to investigate the dyeing potential of Quercus robur L. -
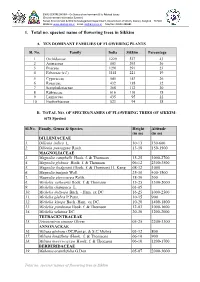
1. Total No. Species/ Name of Flowering Trees in Sikkim
ENVIS CENTRE SIKKIM – On Status of environment & Its Related Issues (Environmental Information System) Forest, Environment & Wildlife Management Department, Government of Sikkim, Deorali, Gangtok – 737102 Website: www.sikenvis.nic.in Email: [email protected] Tele/Fax: 03592-280381 1. Total no. species/ name of flowering trees in Sikkim A. TEN DOMINANT FAMILIES OF FLOWERING PLANTS Sl. No. Family India Sikkim Percentage 1 Orchidaceae 1229 527 43 2 Asteraceae 803 293 36 3 Poaceae 1291 291 23 4 Fabaceae (s.l.) 1141 221 19 5 Cyperaceae 545 143 26 6 Rosaceae 432 138 32 7 Scrophulariaceae 368 112 30 8 Rubiaceae 616 110 18 9 Lamiaceae 435 95 22 10 Euphorbiaceae 523 94 18 B. TOTAL NO. OF SPECIES/NAMES OF FLOWERING TREES OF SIKKIM: (678 Species) Sl.No. Family, Genus & Species Height Altitude (in m) (in m) DILLENIACEAE 1. Dillenia indica L. 10-13 150-600 2. Dillenia pentagyna Roxb. 13-18 150-1500 MAGNOLIACEAE 3. Magnolia campbelli Hook. f. & Thomson 15-25 1000-2700 4. Magnolia globosa Hook. f. & Thomson 06-12 2300-3500 5. Magnolia hodgsonii (Hook. f. & Thomson) H. Keng 08-12 1000 6. Magnolia insignis Wall. 25-30 400-1500 7. Magnolia pterocarpa Roxb. 18-30 500 8. Michelia cathcartii Hook. f. & Thomson 15-25 1500-2000 9. Michelia champaca L. 03-05 10. Michelia doltsopa Buch. - Ham. ex DC. 16-25 1000-2500 11. Michelia glabra P.Pann. 10-15 900 12. Michelia kisopa Buch.-Ham. ex DC. 10-20 1400-1800 13. Michelia punduana Hook. f. & Thomson 12-03 1000-1600 14. Michelia velutina DC.