Development of Novel Carbon Fiber Produced from Waste Fiber by Cabonization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
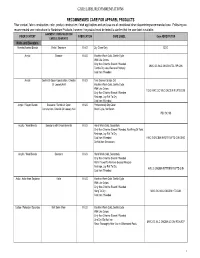
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Textile Printing
TECHNICAL BULLETIN 6399 Weston Parkway, Cary, North Carolina, 27513 • Telephone (919) 678-2220 ISP 1004 TEXTILE PRINTING This report is sponsored by the Importer Support Program and written to address the technical needs of product sourcers. © 2003 Cotton Incorporated. All rights reserved; America’s Cotton Producers and Importers. INTRODUCTION The desire of adding color and design to textile materials is almost as old as mankind. Early civilizations used color and design to distinguish themselves and to set themselves apart from others. Textile printing is the most important and versatile of the techniques used to add design, color, and specialty to textile fabrics. It can be thought of as the coloring technique that combines art, engineering, and dyeing technology to produce textile product images that had previously only existed in the imagination of the textile designer. Textile printing can realistically be considered localized dyeing. In ancient times, man sought these designs and images mainly for clothing or apparel, but in today’s marketplace, textile printing is important for upholstery, domestics (sheets, towels, draperies), floor coverings, and numerous other uses. The exact origin of textile printing is difficult to determine. However, a number of early civilizations developed various techniques for imparting color and design to textile garments. Batik is a modern art form for developing unique dyed patterns on textile fabrics very similar to textile printing. Batik is characterized by unique patterns and color combinations as well as the appearance of fracture lines due to the cracking of the wax during the dyeing process. Batik is derived from the Japanese term, “Ambatik,” which means “dabbing,” “writing,” or “drawing.” In Egypt, records from 23-79 AD describe a hot wax technique similar to batik. -

Material/Source Features Pros Cons Polyester/Cotton Blend 80% Polyester / 20% Cotton, 65/35, and 40/60 Are Common Blends. • La
Material/Source Features Pros Cons Polyester/Cotton Blend Liquid Resistance Appropriate for use in Polyester blends burn Splash resistant. clinical settings and readily when ignited, 80% Polyester / 20% No specific chemical resistance. research laboratories and are not appropriate Cotton, 65/35, and 40/60 Anecdotal evidence suggests polyester blends provide better protection against where biological for use with flammable are common blends. corrosive material than does cotton. material is liquids, pyrophoric Flame Resistance manipulated. materials, or near open No flame. Lab Supply Polyester blends burn more readily than 100% cotton or flame-resistant Companies materials. Chemistry Comfort Stockroom Lightweight and breathable. Biology More cotton in the blend results in better breathability. Stockroom 100% Cotton Liquid Resistance Appropriate for use in Cotton lab coats should Not splash resistant. clinical settings and be supplemented with a Lab Supply No specific chemical resistance. research laboratories chemical splash apron Companies Anecdotal evidence suggests cotton lab coats provide better protection from where there is light when corrosive material Chemistry solvent contamination than corrosive contamination. flammable liquid or is handled. Stockroom Flame Resistance open flame use. Biology No Stockroom Burns less readily than polyester blends. Comfort Lightweight and breathable. 100% Cotton treated with Liquid Resistance Appropriate for use in More costly than a flame retardant. Not splash resistant. research laboratories traditional 100% cotton No specific chemical resistance. where substantial fire lab coat. Lab Supply Anecdotal evidence suggests cotton lab coats provide better protection from risk exists from Companies solvent contamination than corrosive contamination. flammable material Manufacturers of Flame Resistance handling or open flame flame-resistant Yes use. -

Fabric Fiber Content
Fabric Types, Count & Fiber Content Zweigart Linen Count Content Belfast 32 100% linen Afghans - 100% Polyacrylic Cashel 28 100% linen Abby 18ct Alba 14ct Almanac 14ct Cork 19 100% linen Anne Cloth 18ct Baby Snuggle 18ct Country Home 18ct Dublin 25 100% linen Diamond 18ct Gloria 14ct Hearthside 14ct Edinborough 36 100% linen Honeycomb 18ct Novara 14ct Patrice 14ct Fine Linen 45 55% linen + 45% cotton Afghans - 100% Cotton Glasgow 28 100% linen Anne Cloth 18ct Augusta 14 ct Novara 14ct Kingston 50 100% linen Teresa 14ct Newcastle 40 100% linen Afghans- Misc Normandie 55% cotton + 45% linen Pastel LinenD 28 52% cotton + 48% linen Gloria 14ct 70% rayon + 30% linen Pearl Linen 20, 25, 28 60% polyester + 40% linen Merino 28ct 100% Wool Mosaik 18ct 52% cotton + 42% rayon Patterned Count Content Tannenbaum 18ct 52% cotton + 42% rayon Cottage Huck 14 100% cotton Aida Weave Count Content Belinda 20 52% cotton + 48% rayon Diana 20 52% cotton + 48% rayon Aida 8, 11, 14, 16, 18 100% cotton Newport 28 100% linen Country AidaD 7 100% polyacrylic Sambuca 28 60% polyester + 40% linen Damask Aida 11,14,18 52% cotton + 48% rayon Saronno 28 52% cotton + 48% rayon GoldauD 7 55% rayon + Shenandoah 28 55% linen + 45% rayon 40% cotton + 5% metallic Hardanger 22 100% cotton Canvas Count Content Hearthstone 14 60% cotton + 40% linen Congress 24 100% cotton Herta 6 100% cotton Congressa 24 100% cotton Huck 14 100% cotton Cordova 22 100% cotton Klostern 7 60% rayon + 40% cotton Double Mesh 5, 6.5, 7.5, 10, 12, Linen Hardanger 22 100% linen 14, 16, 18, 20 100% cotton -

Fabric Supplier List
FABRIC SUPPLIER LIST CANADA Kendor Textiles Ltd 1260 Cliveden Ave Delta BC V3M 6Y1 Canada 604.434.3233 [email protected] www.kendortextiles.com Fabrics Available: Fabric supplier. Eco-friendly. Organic. Knits: solids, prints, yarn dyes and warp. Wovens: solids and yarn dyes. End Use: activewear, bottomweights, medical, lingerie, childrenswear, swimwear, rainwear, skiwear and uniform. Natural & eco items include cottons, bamboo's, modals, linens, hemps, organic cottons & organic linens. Technical items include waterproof/breathable soft shells, antibacteric & wicking polyester & recycled polyesters. Is a proud representative of the British Millerain line of waxed cottons and wools, and are able to provide custom souring. Minimums: Carries stock. In-stock minimum: 5 yards/color. Minimum order for production: 10 yards/color. Gordon Fabrics LTD #1135-6900 Graybar Rd. Richmond BC Canada 604.275.2672 [email protected] Fabrics Available: Fabric Supplier. Importer. Jobber. Carries stock. Knits & Wovens: solids, prints, yarn dyes and novelties. End Use: activewear, borromweights, eveningwear/bridal, medical, lingerie and childrenswear. Minimums: In stock minimum 1 yard. Minimum order for production varies. StartUp Fashion Supplier List 2016 – Page 1 CHINA Ecopel (HX) Co., Ltd. China +86 216.767.9686 www.ecopel.cn Fabrics Available: Fake fur and leather garments. End Uses: Childrenswear, Menswear, Other, Womenswear. Minimums: Min. order 50-100 m Hangzhou New Design Source Textile Co., Ltd. China +86 057.182.530528 Fabrics Available: Knits, Polyester/Man-Made, Prints. End Uses: Juniors Fashion, Menswear, Womenswear. Minimums: Min order 50 m. Nantong Haukai Textile Co., Ltd. China +86 513.890.78626 www.huakaitex.com Fabrics Available: Cotton, Linen. End Uses: Corporatewear/Suiting, Menswear, Womenswear. -

Eco-Friendly Dyeing of Wool and Pashmina Fabric Using Quercus Robur L. (Fruit Cups) Dye and Salix Alba L
AL SC R IEN TU C A E N F D O N U A N D D Journal of Applied and Natural Science 7 (1) : 138 – 143 (2015) A E I T L I O P JANS N P A ANSF 2008 Eco-friendly dyeing of wool and pashmina fabric using Quercus robur L. (fruit cups) dye and Salix alba L. (wood extract) mordant Syed Maqbool Geelani1*, Shoukat Ara1, Pradeep Kumar Mishra2, S.J.A. Bhat3, Syed Hanifa4, Shamsul Haq1, Iqbal Jeelani5, Gazala Qazi1, Asif H Sofi6, Shakeel A Mir5, P.A. Khan3, Sarfaraz A Wani6 and A S.M. Raja7 1Division of Environmental Sciences, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar-190025 (J&K), INDIA. 2Department of Chemical Engineering & Technology, Indian Institute of Technology (Banaras Hindu University), Varanasi-221005.(UP), INDIA 3Faculty of Forestry, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Wadura-193201, (J&K), INDIA 4Department of Food Sciences & Technology, University of Kashmir, Hazratbal, Srinagar-190006 (J&K), INDIA 5Division of Agricultural Statistics, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar-190025 (J&K), INDIA 6Division of Livestock Products Technology, Faculty of Veterinary Science & Animal Husbandry, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shuhama-190006 (J&K), INDIA 7Division of Textile Manufacture and Textile chemistry, Central Sheep and wool Research Institute, Avikanagar - 304501 (Rajasthan), INDIA *Corresponding author. E-mail: [email protected] Received: November 24, 2014; Revised received: February 20, 2015; Accepted: March 19, 2015 Abstract: Study was conducted to investigate the dyeing potential of Quercus robur L. -

Sonicator Dyeing of Cotton, Wool and Silk with the Leaves Extract
Volume 6, Issue 1, Spring2009 Sonicator Dyeing of Cotton, Wool and Silk with the Leaves Extract Padma S Vankar*a, Rakhi Shanker a, Shalini Dixit a and Debajit Mahantab aFacility for Ecological and Analytical Testing Indian Institute of Technology, Kanpur-208 016, India bArunachal Pradesh State Council for Science & Technology Vivek-Vihar, Itanagar-791 113, Arunachal Pradesh ABSTRACT Malus sikkimensis. (Local name- Chap shaw sheng) belongs to family Rosaceae is being primarily used for preparing tea by Monpas tribes of Arunachal Pradesh. In the present study sonicator dyeing with Malus extract has been demonstrated. Pretreatment with 1-2 % metal mordant and using 5 % of plant extract (owf) was found to be optimum and showed very good hue colors for cotton, wool and silk dyed fabrics. We have demonstrated that the use of sonication in conjunction with metal mordanting has a synergistic effect on better dye uptake, dye adherence and eventually on good fastness properties of the dyed swatches of cotton, silk and wool. Keywords: Malus sikkimensis, natural dye, cotton, wool, silk, commercial dyeing 1. Introduction: Malus sikkimensis, belongs to family Rosaceae, is an evergreen woody tree with a maximum height of 40 m and diameter 2.3 m. It occurs in humid valley forest of Arunachal Pradesh. In the present study Malus leaves (figure-1) have been used as natural dye-stuff, which is primarily used as a substitute for tea by the local Arunachalees. The crude aqueous extract has been shown to dye cotton, silk and wool samples as shown in figures 5-7. Figure-1 Leaves of Malus sikkimensis Traditional Knowledge of its use: Stem and leaves of the plant locally known as 2. -

Digital Textile Printing Opportunities for Sign Companies
Digital Textile Printing Opportunities for Sign Companies This survey remains the property of the International Sign Association. None of the information contained within can be republished without permission from ISA. PREPARED BY: InfoTrends ISA Whitepaper Digital Textile Printing Opportunities for Sign Companies TABLE OF CONTENTS Introduction ......................................................................................................................................2 Key Highlights ..................................................................................................................................2 Recommendations ...........................................................................................................................2 Soft Signage Applications ...............................................................................................................3 The 2014 Textile Industry ................................................................................................................4 Market Growth in Wide Format Digital Printing ...............................................................................5 Technological Shifts ....................................................................................................................5 Application Trends .......................................................................................................................7 Vendors of Graphic Textile and Decorative Solutions .....................................................................7 -

Identifying Woven Textiles 1750-1950 Identification
Identifying Woven Textiles 1750–1950 DATS in partnership with the V&A 1 Identifying Woven Textiles 1750–1950 This information pack has been produced to accompany two one-day workshops taught by Katy Wigley (Director, School of Textiles) and Mary Schoeser (Hon. V&A Senior Research Fellow), held at the V&A Clothworkers’ Centre on 19 April and 17 May 2018. The workshops are produced in collaboration between DATS and the V&A. The purpose of the workshops is to enable participants to improve the documentation and interpretation of collections and make them accessible to the widest audience. Participants will have the chance to study objects at first hand to help increase their confidence in identifying woven textile materials and techniques. This information pack is intended as a means of sharing the knowledge communicated in the workshops with colleagues and the wider public and is also intended as a stand-alone guide for basic weave identification. Other workshops / information packs in the series: Identifying Textile Types and Weaves Identifying Printed Textiles in Dress 1740–1890 Identifying Handmade and Machine Lace Identifying Fibres and Fabrics Identifying Handmade Lace Front Cover: Lamy et Giraud, Brocaded silk cannetille (detail), 1878. This Lyonnais firm won a silver gilt medal at the Paris Exposition Universelle with a silk of this design, probably by Eugene Prelle, their chief designer. Its impact partly derives from the textures within the many-coloured brocaded areas and the markedly twilled cannetille ground. Courtesy Francesca Galloway. 2 Identifying Woven Textiles 1750–1950 Table of Contents Page 1. Introduction 4 2. Tips for Dating 4 3. -

Printing Techniques
PRINTING TECHNIQUES A COMPARISON OF COTTON PRINTING TECHNIQUES Modern textile printing incorporates a wide variety of colorants and technologies to produce a diverse array of printed textile products at various stages in the product development process. Today, rotary screen printing accounts for the majority of cotton printed clothing; however, newer printing technologies such as digital printing and cool transfer printing are attracting attention because of their reduced environmental footprint, and seemingly limitless color and design capabilities. COOL TRANSFER PRINTING DIGITAL PRINTING ROTARY SCREEN PRINTING A process that transfers a print design from paper A process that applies small drops of color through A continuous method of printing in which a to fabric. A special coated paper is printed with the print heads onto a fabric. There is virtually no limit perforated cylindrical screen is used to apply color. desired pattern which is then transferred to the to the number of colors or size of the request. Color is forced from the interior of the screen onto fabric under ambient conditions. the fabric. Cool Transfer Print Digital Print Rotary Screen Print FABRICAST™ SK-1871-14CT FABRICAST™ DK-2810-2A FABRICAST™ SK-1923-4P (Puff) COMPARING PRINTING TYPES = Rotary = Cool Transfer = Digital SPEED PHOTO REALISTIC PRINT PATTERN ESTIMATED WATER, ENERGY, QUALITY FLEXIBILITY COST CHEMICAL INPUTS FAST HIGH HIGH HIGH MOST SLOW LOW LOW LOW LEAST PRINTING TECHNIQUES COOL TRANSFER PRINTING Market interest in transfer printing on cotton fabrics has been strong for many years; however, standard heat transfer printing was achievable only for polyester and nylon fabrics, and the transfer prints available for cotton offered poor hand and were limited in terms of inks available. -

Design and Development of Silk Floss / Cotton Blends Handloom Fabrics
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2015): 78.96 | Impact Factor (2015): 6.391 Design and Development of Silk Floss / Cotton Blends Handloom Fabrics Rashmi Pareet1, Geeta Mahale2 Department of Textile and Apparel Designing, College of Rural Home Science, Dharwad University of Agricultural Sciences, Dharwad-580 005, Karnataka, India Abstract: The present study was carried out to add value to silk floss by developing blended union fabrics. Blending of silk floss and cotton was done by simple stack method and yarns were spun on foot operated medleri charkha. The blend proportions are cotton/silk floss 50:50 and 70:30 and 100 per cent of silk floss yarn were produced. Three union fabrics were also made using pure cotton yarn in warp and blended yarns and pure silk floss in weft. Result revealed that, the cotton x silk floss (control sample) union fabric had highest cloth cover and cotton x cotton/silk floss (70/70) blended union fabric has better dimensional stability, highest thickness and cloth weight. Keywords: Silk floss, blending, union fabrics, geometrical properties 1. Introduction 2. Objectives The textile industry is not only one of the oldest but also To develop blended union handloom fabrics and assess the continues to be one of the main stay of the world economy. geometrical properties of cotton/silk floss blended union The evolution of the textile industry has brought about big fabrics changes in the function of fabric engineering. Today in the Indian scenario, textile industry contributes up to 9 per cent 3. -

Life Cycle Assessment ENVIRONMENTAL PROFILE of COTTON and POLYESTER-COTTON FABRICS
AUTEX Research Journal Vol 1, No.1, 1999, © AUTEX Life Cycle Assessment ENVIRONMENTAL PROFILE OF COTTON AND POLYESTER-COTTON FABRICS Eija M.Kalliala, and Pertti Nousiainen, Tampere University of Technology, PO Box 527, 33101 Tampere, Finland, Europe Abstract The aim of this study was to increase the knowledge of environmental impact associated with producing fabrics for hotel textile services. The project was carried out in two parts: studies on hotel textiles and on textile services in three major Scandinavian laundering companies. This paper presents the results of the hotel textile study. The environmental impact was studied by applying the main principles of the life cycle assessment (LCA) methodology. Life-cycle assessments provide useful information on the quantities of energy and resources consumed and emissions associated with production systems. The impact assessment is still under development but some scenarios have been made to describe possible local, regional and global environmental consequences of the system under study. The inventory calculations proved that cotton fibre production consumes about 40% less energy than polyester fibre production. Cotton growing requires, however, huge amounts of water: irrigated amounts vary from 7 to 29 tons per kg of raw cotton fibres. Pesticides and fertilizers used in traditional cotton cultivation have ecotoxic effects in contrast to organic cotton cultivation, where natural alternatives to agrochemicals are used. It could also be concluded that 50/50 CO/PES sheets in hotel use have fewer environmental impacts than 100% CO sheets. This is due to the higher durability as well as lower laundering energy requirements of 50/50 CO/PES sheets.