Percoidei, Sillaginidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Otolith Studies in Fishes of India
Voyager: Voll. V Dec. 2011, 88-9i: 2011 ,SS,V..097&7436 : INDEXED AND ABSIRACTED 88 REVIEW OF OTOLITH STUDIES + INFISHES OFINDIA Shub\ia Mathur, Seema Jain, Manu Vanna andAnumohini Department Of Zoology,R.GP.G College, Meerut Introduction Otoliths are the most reliable Otoliths are dense calcareous bony ageing structure in a number of fish sfrncture found in the inner ear of fishes. species. There is significant conelation Hearing and balancing functions are between otolith length and weight with carried out by this part. They are the fish size. It is useful to determine the first had part formed in the fi sh and grow age of the candidate species. The continuously by successive deposition knowledge of age and growth of an of mineral-rich calcium carbonate econornically important fi sh is essential (aragonite) and protein-rich layers. for understanding the age composition Otoliths are metabolically inert, not of the stocks and the role of various zubject to reabsorption and remodelling class-years in the fisheries. It is also by grow0r and tlreir ctraracteristic shape essential to determine the mortality and will not be affected by fi sh preservation. sr.rnrival rate ofvarious year-classes and Having these qualities, otoliths proved success of the yearly broods after themselves as good recorders of life recnritnent The age of fistres at different historyofthe fish and its surrounding periods oftheirlives is determined after environment. While the otolith the study ofthe growth rings found in morphology is species-specific, the the otoliths, scales and other bony parts. pattem of growth rings in an otolith Recent studies on otolith helped to microstructure reveals the age and provide a reliable estimate of age temporal growth of the fish in relation to information with accurate and precision the environmental conditions whereas of clear growth pattern in life stages. -

DYNAMIC HABITAT USE of ALBACORE and THEIR PRIMARY PREY SPECIES in the CALIFORNIA CURRENT SYSTEM Calcofi Rep., Vol
MUHLING ET AL.: DYNAMIC HABITAT USE OF ALBACORE AND THEIR PRIMARY PREY SPECIES IN THE CALIFORNIA CURRENT SYSTEM CalCOFI Rep., Vol. 60, 2019 DYNAMIC HABITAT USE OF ALBACORE AND THEIR PRIMARY PREY SPECIES IN THE CALIFORNIA CURRENT SYSTEM BARBARA MUHLING, STEPHANIE BRODIE, MICHAEL JACOX OWYN SNODGRASS, DESIREE TOMMASI NOAA Earth System Research Laboratory University of California, Santa Cruz Boulder, CO Institute for Marine Science Santa Cruz, CA CHRISTOPHER A. EDWARDS ph: (858) 546-7197 Ocean Sciences Department [email protected] University of California, Santa Cruz, CA BARBARA MUHLING, OWYN SNODGRASS, YI XU HEIDI DEWAR, DESIREE TOMMASI, JOHN CHILDERS Department of Fisheries and Oceans NOAA Southwest Fisheries Science Center Delta, British Columbia, Canada San Diego, CA STEPHANIE SNYDER STEPHANIE BRODIE, MICHAEL JACOX Thomas More University, NOAA Southwest Fisheries Science Center Crestview Hills, KY Monterey, CA ABSTRACT peiods, krill, and some cephalopods (Smith et al. 2011). Juvenile north Pacific albacore Thunnus( alalunga) for- Many of these forage species are fished commercially, age in the California Current System (CCS), supporting but also support higher-order predators further up the fisheries between Baja California and British Columbia. food chain, such as other exploited species (e.g., tunas, Within the CCS, their distribution, abundance, and for- billfish) and protected resources (e.g., marine mammals aging behaviors are strongly variable interannually. Here, and seabirds) (Pikitch et al. 2004; Link and Browman we use catch logbook data and trawl survey records to 2014). Effectively managing marine ecosystems to pre- investigate how juvenile albacore in the CCS use their serve these trophic linkages, and improve robustness of oceanographic environment, and how their distributions management strategies to environmental variability, thus overlap with the habitats of four key forage species. -
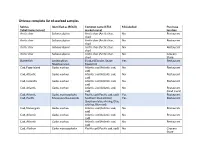
Ottawa: Complete List of Seafood Samples
Ottawa: complete list of seafood samples Sold as Identified as (BOLD) Common name (CFIA Mislabelled Purchase (label/menu/server) market name) location Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Grocery char) Store Butterfish Lepidocybium Escolar (Escolar, Snake Yes Restaurant flavobrunneum Mackerel) Cod, Fogo Island Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) (food truck) Cod, Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod, Pacific Micromesistius australis Southern blue whiting Yes Restaurant (Southern blue whiting, Blue whiting, Blue cod) Cod, Norwegian Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Alaskan Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Pacific Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, North Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Euro Bass Gadus morhua Atlantic cod (Atlantic cod, Yes Restaurant cod) Grouper Epinephelus diacanthus Spinycheek grouper (n/a) Yes – E. -

Yellowfin Tuna
Ahi yellown tuna (Thunnus albacares) is one of two Islands. species known in Hawaii simply as Fishing Methods: intermediaries on all islands, or di- ahi. Similar in general appearance rectly to wholesalers and retailers, or it may be shared with family and to bigeye tuna (the other species - known as ahi friends. Most ahi is sold fresh, but men. A large part of the commercial surpluses caught during the peak be recognized by its more torpedo catch (44%) is harvested by longline shaped body, smaller head and eyes. summer season are sometimes dried boats, which may search for tuna and smoked. In Hawaii, shibi is another name up to 800 nautical miles from port and set hooks in deep waters. Yel- Quality to depths below 600 ft. Landings by either bigeye or albacore tuna. Al- lengthen with age. the island of Hawaii, can be sub- stantial (36%) in some years. Troll- Seasonality & How ers contribute most of the remain- does not retain the beautiful natu- They Are Caught der (20%) of the commercial catch ral red color as long as bigeye. The - Availability and Seasonality: - Caught year-round in Hawaii’s wa- ing tournaments held in Hawaii. method, care in handling and other Distribution: abundant during the summer sea- The longline catch and some of the son (May-September). There are handline (ika-shibi) catch of ahi is species. Noticeable changes occur auction. The majority of the hand- Hawaii. ocean surface temperatures and line catch is sold to wholesalers and other oceanographic conditions fa- intermediary buyers on the island of surface during the summer season vor the migration of ahi schools to are susceptible to a quality defect The troll catch may be marketed known as “burnt tuna”. -
Albacore Tuna Have fl Uctuated Considerably from Year To
Tuna [211] 86587_p211_220.indd 211 12/30/04 4:53:37 PM highlights ■ The catches of Pacifi c bluefi n tuna and North Pacifi c albacore tuna have fl uctuated considerably from year to Ocean year, but no upward or downward trends are apparent for either species. and ■ Increasing the age at entry of Pacifi c bluefi n into the fi shery might increase the yields per recruit of that Climate species. ■ The status of North Pacifi c albacore is uncertain, but most scientists believe that greater harvests of that species Changes would not be sustainable. [212] 86587_p211_220.indd 212 12/30/04 4:53:38 PM background The Inter-American Tropical Tuna Commission (IATTC) studies the tunas of the eastern Pacifi c Ocean (EPO), defi ned for its purposes as the area bounded by the coastline of North, Central, and South America, 40ºN, 150ºW, and 40ºS. The IATTC staff maintains records for most of the vessels that fi sh at the surface for skipjack tuna (Katsuwonus pelamis), yellowfi n tuna (Thunnus albacares), bigeye tuna (T. obesus), and Pacifi c bluefi n tuna (T. orientalis) in the EPO. Pacifi c bluefi n and albacore tuna (T. alalunga) are the tunas most relevant to the region of interest to PICES. Pacifi c bluefi n tuna Spawning of Pacifi c bluefi n apparently takes place only Age-1 and older fi sh are caught by purse seining, in the western Pacifi c Ocean (WPO). Some juvenile mostly during May-September between about 30°- bluefi n move from the WPO to the EPO, and then later 42°N and 140°-152°E. -

Seafood Guide
eat It’s good for you! What pregnant and breastfeeding women and parents of young children need to know. Fish are nutritious and most are very How can you safely safe to eat. eat fish? • Fish have protein and healthy fats, called omega-3s, which are not • Eat a variety of fish that are lower found in other meats. in mercury. • Omega-3s are good for your heart • Eat the amounts of fish shown on and brain. the other side of this pamphlet. • The nutrients in fish are especially • Eat only the flesh or meat of important as your baby develops the fish. Throw away the bones, during pregnancy, throughout head, guts, fat, and skin. breastfeeding, and as your young • Avoid shark, swordfish, tilefish, or child grows. king mackerel. They are highest in • Some fish may contain a chemical mercury. called mercury. Too much mercury • Avoid raw and undercooked in your diet can be harmful. It’s fish and shellfish. best to eat fish that are lower in mercury. For more information about mercury in your fish, visit the Environmental Protection Agency — Fish Advisory at www.epa.gov/choose-fish-and-shellfish-wisely. choose safe Follow these tips to enjoy the health benefits of eating fish low in mercury and high in omega-3s. 1. Safe to Eat 2. Do Not Eat Eat fish from the list below 2 to 3 These fish are high in mercury. times a week. Choose fish from stores • Shark • King Mackerel or restaurants. • Swordfish • Tilefish • For women, eat about 8 to 12 ounces a week total. -

(2): 375-385 the Fishes of the Family Sillaginidae from India with a Description of a New Spec
/. mar. biol Ass, India, 1976, 18 (2): 375-385 THE FISHES OF THE FAMILY SILLAGINIDAE FROM INDIA WITH A DESCRIPTION OF A NEW SPECIES* RoLAKD J. MCKAY Queensland Museum, Brisbane. Australia ABSTRACT A review of the fishes of the family Sillaginidae from Indian waters is given with a description of a new species of Siliago. A key to the genera and species is provided. INTRODUCTION TUB sandborers or Indo-Paciiic sillagos of the family Sillaginidae are popular food fishes captured by seine-net, cast-net. Gill-net, lift-net and trawl-net in inshore and estuarine waters of the Indo-Pacific region from South Africa to northern Japan. The species are most numerous in Australian waters and are being reviewed by the author. In India they are known by a variety of local names and form a small but important fishery. I am deeply grateful to Dr. W. Fischer of the Fisheries Resources Service of FAG, Rome, for the opportunity to participate in the FAO/DANIDA Expert Consultation Programme at the Central Marine Fisheries Research Institute, Cochin, 1980. During this Programme many sillaginids were examined and Siliago vincenti discovered. My colleagues at Cochin assisted me in collecting and photographing the new species. Dr. E. G. Silas, Director, CMFRI, Cochin made early publica tion of this paper possible and made facilities available for the examination and illustration of specimens. To the staff of CMFRI, especially Mr. A, Noble and the artist Mr. A. Muniyandhi, my appreciation for their generous assistance. To my friend Mr. S; G. Vincent of CMFRI my thanks for his valuablp assistance in coUectirig specimens, obtaining information for this study recognising the two species in the field and assisting with the measurements. -

Biodiversity Action Plan Full Report
Final Report Project Code 2012MC09 Biodiversity Action Plan For Malvan and Devgad Blocks, Sindhudurg District, Maharashtra Prepared for Mangrove Cell, GoM i Conducting Partipicatory Rural Appraisal in the Coastal Villages of SIndhudurg District © The Energy and Resources Institute 2013 Suggested format for citation T E R I. 2013 Participatory Rural Appraisal Study in Devgad and Malvan Blocks, Sindhudurg District New Delhi: The Energy and Resources Institute 177 pp. For more information Dr. Anjali Parasnis Associate Director, Western Regional Centre Tel: 022 27580021/ 40241615 The Energy and Resources Institute E-mail: [email protected] 318, Raheja Arcade, sector 11, Fax: 022-27580022 CBD-Belapur, Navi Mumbai - 400 614, India Web: www.teriin.org ii Conducting Partipicatory Rural Appraisal in the Coastal Villages of SIndhudurg District Contents Abbrevations: .......................................................................................................................... x Executive Summary ............................................................................................................. xii 1. SINDHUDURG: AN INTRODUCTION .................................................................................... 14 1.1 Climate and rainfall: ...................................................................................................... 15 1.2 Soil: ................................................................................................................................... 15 1.3 Cropping pattern:.......................................................................................................... -

Relationship Between Otolith Morphometry and Fish Size of Otolithoides Pama (Hamilton, 1822) from Hooghly-Matlah Estuary, India
Indian Journal of Geo Marine Sciences Vol. 49 (10), October 2020, pp. 1636-1642 Relationship between otolith morphometry and fish size of Otolithoides pama (Hamilton, 1822) from Hooghly-Matlah estuary, India D Bhakta*,a,b, S K Dasa, B K Dasb, S Beheraa & T S Nagesha aDepartment of Fisheries Resource Management, Faculty of Fishery Sciences, WBUAFS, Chakgaria, Kolkata, West Bengal – 700 094, India bICAR-Central Inland Fisheries Research Institute, Barrackpore, Monirampore, Kolkata, West Bengal – 700 120, India *[E-mail: [email protected]] Received 04 November 2018; revised 06 August 2020 The correlation between sagitta otolith morphometry (length, weight, and breadth) and weight of Otolithoides pama (Hamilton, 1822) occurring in the Hooghly-Matlah estuary of West Bengal was examined for one year (February 2017 to January 2018). The sagitta otoliths were extracted, cleaned, photographed, and measured. Otolith length, weight, and breadth were recorded for each pair of sagittae. The length and weight of the fish sample, as well as those of otoliths, ranged from 51 to 327 mm, 1.1 to 270 g, 2.0 to 13.9 mm, and 0.0085 to 0.756 g, respectively. A linear relationship existed between the length and weight of otolith with the length of fish. The relationship between total fish length (TL) and otolith length (OL) was recorded as TL = 0.038 (OL) + 0.123 (R2 = 0.799), that of total fish length (TL) and otolith weight (OW) being TL = 0.025 (OW) - 0.221 (R2 = 0.887), that of total fish length (TL) and otolith breadth being TL = 0.031 (OB) + 0.089 (R2 = 0.781). -

Doctor of Vbilozopbp L1
STUDIES ON SOME ASPECTS OF BIOLOGY OF SELECTED FRESHWATER TELEOST FROM RIVER GANGA THESIS SUBMITTED FOR THE AWARD OF THE DEGREE OF ]Doctor of Vbilozopbp IN ZOOLOGY Submitted By SHAHISTA KHAN Under the Supervision of DR. MOHAMMAD AFZAL KHAN SECTION OF FISHERY SCIENCE AND AQUACULTURE DEPARTMENT OF ZOOLOGY ALIGARH MUSLIM UNIVERSITY ALIGARH (INDIA) :- I 2013 L1 Phone (External 2700920121-3430 l Internal 3430, 3431 DEPARTMENT OF ZOOLOGY ALIGARH MUSLIM UNIVERSITY ALIGARH - 202 002 INDIA ctions 1 ENTOMOLOGY £/FISHERY SCIENCE & AQUACULTURE D. ......................./No ZO ....................: 3 GENETICS 4 NEMATOLOGY 5 PARASITOLOGY Dated..QL:10.,2-013 ........................... CERTIFICATE This is to certify that the thesis entitled "Studies on some aspects of biology of selected freshwater teleost fronifromriver Ganga" has been completed under my supervision by Ms. Shahista Khan. The work is original and independently pursued by the candidate. It embodies some interesting observations contributing to the existing knowledge on the subject. I permit the candidate to submit the thesis for the award of PhD Degree in Zoology. (Or. Mofiammad'Afza(Kjan) Assistant cProfessor ACKNOWLEDGEMENTS first and foremost I express rrry profound sense of fionour and gratitude to 'TheAGmighty fLLA2Cfor'1is great mercy and choicest blessings geuerous(y bestowed upon Inc. without which I caul( have never seer the completion of ray ref.(D. work I awe my deepest gratitude to my supervisor, Dr. MohammadAf a( 7(han for his keen interest, skiCCf rlguidmug va(ua6(e suggestions, kindness and tumefy lie1p during the entire study programme that fed to preparation of this manuscript. I am thaukr( to cProf. Irfan AGmad, Chairman, rDepartmeat of Zoology, Aligarh sLlustm 'University, A(igar6, for al/biting me to avail the necessary laboratory and(i6rary facilities to carry out the worksuccessful(y. -

C1. Tuna and Tuna-Like Species
163 C1. TUNA AND TUNA-LIKE SPECIES exceptional quality reached US$500 per kg and by Jacek Majkowski * more recently even more, but such prices referring to very few single fish do not reflect the INTRODUCTION situation with the market. Bigeye are also well priced on the sashimi markets. Although The sub-order Scombroidei is usually referred to yellowfin are also very popular on these markets, as tuna and tuna-like species (Klawe, 1977; the prices they bring are much lower. For Collette and Nauen, 1983; Nakamura, 1985). It is canning, albacore fetch the best prices due to composed of tunas (sometimes referred to as true their white meat, followed by yellowfin and tunas), billfishes and other tuna-like species. skipjack for which fishermen are paid much less They include some of the largest and fastest than US$1 per kg. The relatively low prices of fishes in the sea. canning-quality fish are compensated by their The tunas (Thunnini) include the most very large catches, especially in the case of economically important species referred to as skipjack and yellowfin. Longtail tuna principal market tunas because of their global (T. tonggol) is becoming increasingly important economic importance and their intensive for canning and the subject of substantial international trade for canning and sashimi (raw international trade. The consumption of tuna and fish regarded as delicacy in Japan and tuna-like species in forms other than canned increasingly, in several other countries). In fact, products and sashimi is increasing. the anatomy of some tuna species seems to have The tunas other than the principal market species been purpose-designed for canning and loining. -

ILLEGAL FISHING Which Fish Species Are at Highest Risk from Illegal and Unreported Fishing?
ILLEGAL FISHING Which fish species are at highest risk from illegal and unreported fishing? October 2015 CONTENTS EXECUTIVE SUMMARY 3 INTRODUCTION 4 METHODOLOGY 5 OVERALL FINDINGS 9 NOTES ON ESTIMATES OF IUU FISHING 13 Tunas 13 Sharks 14 The Mediterranean 14 US Imports 15 CONCLUSION 16 CITATIONS 17 OCEAN BASIN PROFILES APPENDIX 1: IUU Estimates for Species Groups and Ocean Regions APPENDIX 2: Estimates of IUU Risk for FAO Assessed Stocks APPENDIX 3: FAO Ocean Area Boundary Descriptions APPENDIX 4: 2014 U.S. Edible Imports of Wild-Caught Products APPENDIX 5: Overexploited Stocks Categorized as High Risk – U.S. Imported Products Possibly Derived from Stocks EXECUTIVE SUMMARY New analysis by World Wildlife Fund (WWF) finds that over 85 percent of global fish stocks can be considered at significant risk of Illegal, Unreported, and Unregulated (IUU) fishing. This evaluation is based on the most recent comprehensive estimates of IUU fishing and includes the worlds’ major commercial stocks or species groups, such as all those that are regularly assessed by the United Nations Food and Agriculture Organization (FAO). Based on WWF’s findings, the majority of the stocks, 54 percent, are categorized as at high risk of IUU, with an additional 32 perent judged to be at moderate risk. Of the 567 stocks that were assessed, the findings show that 485 stocks fall into these two categories. More than half of the world’s most overexploited stocks are at the highest risk of IUU fishing. Examining IUU risk by location, the WWF analysis shows that in more than one-third of the world’s ocean basins as designated by the FAO, all of these stocks were at high or moderate risk of IUU fishing.