8.2 the Significance of Ocean Deoxygenation for Open Ocean Tunas and Billfishes Shirley Leung,K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FISH LIST WISH LIST: a Case for Updating the Canadian Government’S Guidance for Common Names on Seafood
FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood Authors: Christina Callegari, Scott Wallace, Sarah Foster and Liane Arness ISBN: 978-1-988424-60-6 © SeaChoice November 2020 TABLE OF CONTENTS GLOSSARY . 3 EXECUTIVE SUMMARY . 4 Findings . 5 Recommendations . 6 INTRODUCTION . 7 APPROACH . 8 Identification of Canadian-caught species . 9 Data processing . 9 REPORT STRUCTURE . 10 SECTION A: COMMON AND OVERLAPPING NAMES . 10 Introduction . 10 Methodology . 10 Results . 11 Snapper/rockfish/Pacific snapper/rosefish/redfish . 12 Sole/flounder . 14 Shrimp/prawn . 15 Shark/dogfish . 15 Why it matters . 15 Recommendations . 16 SECTION B: CANADIAN-CAUGHT SPECIES OF HIGHEST CONCERN . 17 Introduction . 17 Methodology . 18 Results . 20 Commonly mislabelled species . 20 Species with sustainability concerns . 21 Species linked to human health concerns . 23 Species listed under the U .S . Seafood Import Monitoring Program . 25 Combined impact assessment . 26 Why it matters . 28 Recommendations . 28 SECTION C: MISSING SPECIES, MISSING ENGLISH AND FRENCH COMMON NAMES AND GENUS-LEVEL ENTRIES . 31 Introduction . 31 Missing species and outdated scientific names . 31 Scientific names without English or French CFIA common names . 32 Genus-level entries . 33 Why it matters . 34 Recommendations . 34 CONCLUSION . 35 REFERENCES . 36 APPENDIX . 39 Appendix A . 39 Appendix B . 39 FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood 2 GLOSSARY The terms below are defined to aid in comprehension of this report. Common name — Although species are given a standard Scientific name — The taxonomic (Latin) name for a species. common name that is readily used by the scientific In nomenclature, every scientific name consists of two parts, community, industry has adopted other widely used names the genus and the specific epithet, which is used to identify for species sold in the marketplace. -

Striped Marlin (Kajikia Audax)
I & I NSW WILD FISHERIES RESEARCH PROGRAM Striped Marlin (Kajikia audax) EXPLOITATION STATUS UNDEFINED Status is yet to be determined but will be consistent with the assessment of the south-west Pacific stock by the Scientific Committee of the Central and Western Pacific Fisheries Commission. SCIENTIFIC NAME STANDARD NAME COMMENT Kajikia audax striped marlin Previously known as Tetrapturus audax. Kajikia audax Image © I & I NSW Background lengths greater than 250cm (lower jaw-to-fork Striped marlin (Kajikia audax) is a highly length) and can attain a maximum weight of migratory pelagic species distributed about 240 kg. Females mature between 1.5 and throughout warm-temperate to tropical 2.5 years of age whilst males mature between waters of the Indian and Pacific Oceans.T he 1 and 2 years of age. Striped marlin are stock structure of striped marlin is uncertain multiple batch spawners with females shedding although there are thought to be separate eggs every 1-2 days over 4-41 events per stocks in the south-west, north-west, east and spawning season. An average sized female of south-central regions of the Pacific Ocean, as about 100 kg is able to produce up to about indicated by genetic research, tagging studies 120 million eggs annually. and the locations of identified spawning Striped marlin spend most of their time in grounds. The south-west Pacific Ocean (SWPO) surface waters above the thermocline, making stock of striped marlin spawn predominately them vulnerable to surface fisheries.T hey are during November and December each year caught mostly by commercial longline and in waters warmer than 24°C between 15-30°S recreational fisheries throughout their range. -

A Valuation of Tuna in the Eastern Pacific Ocean
A fact sheet from July 2016 Gerard Soury Netting Billions: A Valuation of Tuna in the Eastern Pacific Ocean Overview Together, the seven most commercially important tuna species are among the most economically valuable fish on the planet. Globally, commercially landed tuna product has a value of US$10 billion to $12 billion per year to the fishermen that target tunas and more than US$42 billion per year at the final point of sale. These conservative totals do not account for noncommercial values of tuna, including those associated with sport fishing, other forms of tourism, and the ecosystem benefits of living tunas. Figure 1 Value of Tuna in the Eastern Pacific by Species Though second to skipjack in landings, yellowfin lead the way in value for fishermen and at the final point of sale Landings (metric tons) 262,000 95,000 243,000 48,000 5,000 Dock value (USD) $0.3 billion $0.29 billion $0.33 billion $0.13 billion $0.063 billion End value (USD) $1.67 billion $2.4 billion $0.43 billion $0.99 billion $0.3 billion Skipjack Albacore Bigeye Yellowfin Pacific bluefin Note: Landings, dock value, and end value in 2014 of tuna caught in the IATTC Convention Area, based on data provided by fishing nations to the IATTC and a market analysis performed by Poseidon Aquatic Resource Management Ltd. Source: Graeme Macfadyen, Estimate of Global Sales Values From Tuna Fisheries—Phase 3 Report, Poseidon Aquatic Resource Management Ltd. (2016) © 2016 The Pew Charitable Trusts The Pacific Ocean is the source of about 70 percent of commercially landed tuna and 65 to 70 percent of the global value of tuna at both the dock and the final point of sale. -

Do Some Atlantic Bluefin Tuna Skip Spawning?
SCRS/2006/088 Col. Vol. Sci. Pap. ICCAT, 60(4): 1141-1153 (2007) DO SOME ATLANTIC BLUEFIN TUNA SKIP SPAWNING? David H. Secor1 SUMMARY During the spawning season for Atlantic bluefin tuna, some adults occur outside known spawning centers, suggesting either unknown spawning regions, or fundamental errors in our current understanding of bluefin tuna reproductive schedules. Based upon recent scientific perspectives, skipped spawning (delayed maturation and non-annual spawning) is possibly prevalent in moderately long-lived marine species like bluefin tuna. In principle, skipped spawning represents a trade-off between current and future reproduction. By foregoing reproduction, an individual can incur survival and growth benefits that accrue in deferred reproduction. Across a range of species, skipped reproduction was positively correlated with longevity, but for non-sturgeon species, adults spawned at intervals at least once every two years. A range of types of skipped spawning (constant, younger, older, event skipping; and delays in first maturation) was modeled for the western Atlantic bluefin tuna population to test for their effects on the egg-production-per-recruit biological reference point (stipulated at 20% and 40%). With the exception of extreme delays in maturation, skipped spawning had relatively small effect in depressing fishing mortality (F) threshold values. This was particularly true in comparison to scenarios of a juvenile fishery (ages 4-7), which substantially depressed threshold F values. Indeed, recent F estimates for 1990-2002 western Atlantic bluefin tuna stock assessments were in excess of threshold F values when juvenile size classes were exploited. If western bluefin tuna are currently maturing at an older age than is currently assessed (i.e., 10 v. -

Fao Species Catalogue
FAO Fisheries Synopsis No. 125, Volume 5 FIR/S125 Vol. 5 FAO SPECIES CATALOGUE VOL. 5. BILLFISHES OF THE WORLD AN ANNOTATED AND ILLUSTRATED CATALOGUE OF MARLINS, SAILFISHES, SPEARFISHES AND SWORDFISHES KNOWN TO DATE UNITED NATIONS DEVELOPMENT PROGRAMME FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS FAO Fisheries Synopsis No. 125, Volume 5 FIR/S125 Vol.5 FAO SPECIES CATALOGUE VOL. 5 BILLFISHES OF THE WORLD An Annotated and Illustrated Catalogue of Marlins, Sailfishes, Spearfishes and Swordfishes Known to date MarIins, prepared by Izumi Nakamura Fisheries Research Station Kyoto University Maizuru Kyoto 625, Japan Prepared with the support from the United Nations Development Programme (UNDP) UNITED NATIONS DEVELOPMENT PROGRAMME FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome 1985 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory. city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-42 ISBN 92-5-102232-1 All rights reserved . No part of this publicatlon may be reproduced. stored in a retriewal system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwase, wthout the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations Via delle Terme di Caracalla, 00100 Rome, Italy. -

DYNAMIC HABITAT USE of ALBACORE and THEIR PRIMARY PREY SPECIES in the CALIFORNIA CURRENT SYSTEM Calcofi Rep., Vol
MUHLING ET AL.: DYNAMIC HABITAT USE OF ALBACORE AND THEIR PRIMARY PREY SPECIES IN THE CALIFORNIA CURRENT SYSTEM CalCOFI Rep., Vol. 60, 2019 DYNAMIC HABITAT USE OF ALBACORE AND THEIR PRIMARY PREY SPECIES IN THE CALIFORNIA CURRENT SYSTEM BARBARA MUHLING, STEPHANIE BRODIE, MICHAEL JACOX OWYN SNODGRASS, DESIREE TOMMASI NOAA Earth System Research Laboratory University of California, Santa Cruz Boulder, CO Institute for Marine Science Santa Cruz, CA CHRISTOPHER A. EDWARDS ph: (858) 546-7197 Ocean Sciences Department [email protected] University of California, Santa Cruz, CA BARBARA MUHLING, OWYN SNODGRASS, YI XU HEIDI DEWAR, DESIREE TOMMASI, JOHN CHILDERS Department of Fisheries and Oceans NOAA Southwest Fisheries Science Center Delta, British Columbia, Canada San Diego, CA STEPHANIE SNYDER STEPHANIE BRODIE, MICHAEL JACOX Thomas More University, NOAA Southwest Fisheries Science Center Crestview Hills, KY Monterey, CA ABSTRACT peiods, krill, and some cephalopods (Smith et al. 2011). Juvenile north Pacific albacore Thunnus( alalunga) for- Many of these forage species are fished commercially, age in the California Current System (CCS), supporting but also support higher-order predators further up the fisheries between Baja California and British Columbia. food chain, such as other exploited species (e.g., tunas, Within the CCS, their distribution, abundance, and for- billfish) and protected resources (e.g., marine mammals aging behaviors are strongly variable interannually. Here, and seabirds) (Pikitch et al. 2004; Link and Browman we use catch logbook data and trawl survey records to 2014). Effectively managing marine ecosystems to pre- investigate how juvenile albacore in the CCS use their serve these trophic linkages, and improve robustness of oceanographic environment, and how their distributions management strategies to environmental variability, thus overlap with the habitats of four key forage species. -

Atlantic Bluefin Tuna (Thunnus Thynnus) Population Dynamics
Environ. Sci. Technol. 2009, 43, 8522–8527 nations of the International Commission for the Conservation Atlantic Bluefin Tuna (Thunnus of Atlantic Tunas (ICCAT) currently manage ABFT fisheries thynnus) Population Dynamics assuming two units (a western stock spawning in the Gulf of Mexico, and an eastern stock which spawns in the Delineated by Organochlorine Mediterranean Sea) ostensibly separated by the 45° W meridian with little intermixing between stocks. However, Tracers tagging studies indicate that bluefin tuna undergo extensive and complex migrations, including trans-Atlantic migrations, ,† and that stock mixing could be as high as 30% (2-4). Extensive REBECCA M. DICKHUT,* - ASHOK D. DESHPANDE,‡ mixing of eastern and western stocks (35 57% bluefin tuna ALESSANDRA CINCINELLI,§ of eastern origin) within the U.S. Mid Atlantic Bight was also 18 MICHELE A. COCHRAN,† reported recently based on otolith δ O values (5). The SIMONETTA CORSOLINI,| uncertainty of stock structures due to mixing makes it difficult RICHARD W. BRILL,† DAVID H. SECOR,⊥ for fisheries managers to assess the effectiveness of rebuilding AND JOHN E. GRAVES† efforts for the dwindling western Atlantic spawning stock of Virginia Institute of Marine Science, Gloucester Point, bluefin tuna. Understanding ABFT spatial distributions and Virginia 23062, National Marine Fisheries Service, dynamics are vital for robust population assessments and Highlands, New Jersey 07732, Department of Chemistry, the design of effective management strategies, and there is University of Florence, 50019 -

Black Marlin (Makaira Indica) Blue Marlin (Makaira Nigricans) Blue
Black marlin (Makaira indica) Blue marlin (Makaira nigricans) Blue shark (Prionace glauca) Opah (Lampris guatus) Shorin mako shark (Isurus oxyrinchus) Striped marlin (Kaijkia audax) Western Central Pacific, North Pacific, South Pacific Pelagic longline July 12, 2016 Alexia Morgan, Consulng Researcher Disclaimer Seafood Watch® strives to have all Seafood Reports reviewed for accuracy and completeness by external sciensts with experse in ecology, fisheries science and aquaculture. Scienfic review, however, does not constute an endorsement of the Seafood Watch® program or its recommendaons on the part of the reviewing sciensts. Seafood Watch® is solely responsible for the conclusions reached in this report. Table of Contents Table of Contents 2 About Seafood Watch 3 Guiding Principles 4 Summary 5 Final Seafood Recommendations 5 Introduction 8 Assessment 10 Criterion 1: Impacts on the species under assessment 10 Criterion 2: Impacts on other species 22 Criterion 3: Management Effectiveness 45 Criterion 4: Impacts on the habitat and ecosystem 55 Acknowledgements 58 References 59 Appendix A: Extra By Catch Species 69 2 About Seafood Watch Monterey Bay Aquarium’s Seafood Watch® program evaluates the ecological sustainability of wild-caught and farmed seafood commonly found in the United States marketplace. Seafood Watch® defines sustainable seafood as originang from sources, whether wild-caught or farmed, which can maintain or increase producon in the long-term without jeopardizing the structure or funcon of affected ecosystems. Seafood Watch® makes its science-based recommendaons available to the public in the form of regional pocket guides that can be downloaded from www.seafoodwatch.org. The program’s goals are to raise awareness of important ocean conservaon issues and empower seafood consumers and businesses to make choices for healthy oceans. -
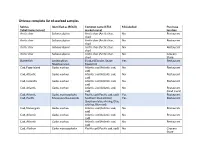
Ottawa: Complete List of Seafood Samples
Ottawa: complete list of seafood samples Sold as Identified as (BOLD) Common name (CFIA Mislabelled Purchase (label/menu/server) market name) location Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Grocery char) Store Butterfish Lepidocybium Escolar (Escolar, Snake Yes Restaurant flavobrunneum Mackerel) Cod, Fogo Island Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) (food truck) Cod, Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod, Pacific Micromesistius australis Southern blue whiting Yes Restaurant (Southern blue whiting, Blue whiting, Blue cod) Cod, Norwegian Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Alaskan Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Pacific Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, North Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Euro Bass Gadus morhua Atlantic cod (Atlantic cod, Yes Restaurant cod) Grouper Epinephelus diacanthus Spinycheek grouper (n/a) Yes – E. -

NOAA's Description of the U.S Commercial Fisheries Including The
6.0 DESCRIPTION OF THE PELAGIC LONGLINE FISHERY FOR ATLANTIC HMS The HMS FMP provides a thorough description of the U.S. fisheries for Atlantic HMS, including sectors of the pelagic longline fishery. Below is specific information regarding the catch of pelagic longline fishermen in the Gulf of Mexico and off the Southeast coast of the United States. For more detailed information on the fishery, please refer to the HMS FMP. 6.1 Pelagic Longline Gear The U.S. pelagic longline fishery for Atlantic HMS primarily targets swordfish, yellowfin tuna, or bigeye tuna in various areas and seasons. Secondary target species include dolphin, albacore tuna, pelagic sharks including mako, thresher, and porbeagle sharks, as well as several species of large coastal sharks. Although this gear can be modified (i.e., depth of set, hook type, etc.) to target either swordfish, tunas, or sharks, like other hook and line fisheries, it is a multispecies fishery. These fisheries are opportunistic, switching gear style and making subtle changes to the fishing configuration to target the best available economic opportunity of each individual trip. Longline gear sometimes attracts and hooks non-target finfish with no commercial value, as well as species that cannot be retained by commercial fishermen, such as billfish. Pelagic longline gear is composed of several parts. See Figure 6.1. Figure 6.1. Typical U.S. pelagic longline gear. Source: Arocha, 1997. When targeting swordfish, the lines generally are deployed at sunset and hauled in at sunrise to take advantage of the nocturnal near-surface feeding habits of swordfish. In general, longlines targeting tunas are set in the morning, deeper in the water column, and hauled in the evening. -
Status of Billfish Resources and the Billfish Fisheries in the Western
SLC/FIAF/C1127 (En) FAO Fisheries and Aquaculture Circular ISSN 2070-6065 STATUS OF BILLFISH RESOURCES AND BILLFISH FISHERIES IN THE WESTERN CENTRAL ATLANTIC Source: ICCAT (2015) FAO Fisheries and Aquaculture Circular No. 1127 SLC/FIAF/C1127 (En) STATUS OF BILLFISH RESOURCES AND BILLFISH FISHERIES IN THE WESTERN CENTRAL ATLANTIC by Nelson Ehrhardt and Mark Fitchett School of Marine and Atmospheric Science, University of Miami Miami, United States of America FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Bridgetown, Barbados, 2016 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-109436-5 © FAO, 2016 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate DFNQRZOHGJHPHQWRI)$2DVWKHVRXUFHDQGFRS\ULJKWKROGHULVJLYHQDQGWKDW)$2¶VHQGRUVHPHQWRI XVHUV¶YLHZVSURGXFWVRUVHUYLFHVLVQRWLPSOLHGLQDQ\ZD\ All requests for translation and adaptation rights, and for resale and other commercial use rights should be made via www.fao.org/contact-us/licence-request or addressed to [email protected]. -

Bermuda's Marine Reserve
POTENTIAL IMPACTS OF BERMUDA’s MARINE RESERVE ON SPORTFISHING TOURISM The Need to Explore Other Options ECONOMIC RETURNS CAN FLOW TO COASTAL NATIONS that Implement Billfish Conservation Measures Sportfishing eco-tourism is a strong economic driver for communities. Visting anglers are driven by the likelihood of catching a lot of billfish. Anticipated quality of a billfishing trip greatly influences anglers’ destination choices. Anglers most often select fishing destination where commercial fishing is restrained. Responsible management of billfish can maximize catch rates and economic returns to communities. As of 2011, more than 3.2 created Of the visitors who million anglers fished outside visited Panama that did not of the United States (exclud- fish, 30% said they would ing Canada) at least once in be interested in fishing on a the previous five years. Anglers subsequent trip to Panama. have many options when it comes to where they travel to fish, and countries must ISLA MUJERES, give particular attention to the socio-economic benefits MEXICO - Marlin and generated from sportfishing Sport Fishing magazines, both when making decisions that international publications could impact their sportfish- Photo courtesy of Viljoen with widespread circulation in ing industry. With only 0.3% the sportfishing community of these anglers reported to recognized Isla Mujeres, have taken their last fishing Mexico on the Yucatan trip to Bermuda, there is peninsula as being one of great potential for increasing the “most desired sailfishing tourism from sportfishing in destinations.” Marlin rated Bermuda.1 Isla as Number Three among its “Easy Billfish Destinations” to fish for Atlantic sailfish.Sport Fishing magazine rated Isla Mujeres in their “Top 20 Sailfish Hot Spots” of the world.