Discriminant Analysis of Colour Measurements Reveals Allele Dosage Effect of ASIP/MC1R in Bay Horses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I . the Color Gene C
THE ABC OF COLOR INHERITANCE IN HORSES W. E. CASTLE Division of Genetics, University of California, Berkeley, California Received October, 27, 1947 HE study of color inheritance in horses was begun in the early days of Tgenetics. Indeed many facts concerning it had already been established earlier, by DARWINin his book on “Variation of Animals and Plants under Domestication.” At irregular inteivals since then, new attempts have been made to collect and classify in terms of genetic factors the records contained in stud books concerning the colors of colts in relation to the colors of their sires and dams. A full bibliography is given by CREWand BuCHANAN-SMITH (19301. By such studies, we have acquired very full information as to what color a colt may be expected to have, when the color of its parents and grandparents is known. This knowledge is empirical rather than experimental in nature. For horses being slow breeding and expensive are rarely available for direct experi- mental study, such as can be made with the small laboratory mammals, mice, rats, rabbits and guinea pigs. We have definite information that color inheritance in horses involves the existence of mutant genes similar to those demonstrated by experimental studies to be involved in color inheritance of other mammals. But the horse genes have been given special names, as they were successively discovered, and it is difficult at present to correlate them with the better known names and geneic symbols used by the experimental breeders. The present paper is an attempt to make such a correlation. Just as in morphological studies comparative anatomy was found useful and still is used to establish homologies between systems of organs, so in mammalian genetics, a comparative study of gene action in the production of coat colors and color patterns may also be of value. -

List of Horse Breeds 1 List of Horse Breeds
List of horse breeds 1 List of horse breeds This page is a list of horse and pony breeds, and also includes terms used to describe types of horse that are not breeds but are commonly mistaken for breeds. While there is no scientifically accepted definition of the term "breed,"[1] a breed is defined generally as having distinct true-breeding characteristics over a number of generations; its members may be called "purebred". In most cases, bloodlines of horse breeds are recorded with a breed registry. However, in horses, the concept is somewhat flexible, as open stud books are created for developing horse breeds that are not yet fully true-breeding. Registries also are considered the authority as to whether a given breed is listed as Light or saddle horse breeds a "horse" or a "pony". There are also a number of "color breed", sport horse, and gaited horse registries for horses with various phenotypes or other traits, which admit any animal fitting a given set of physical characteristics, even if there is little or no evidence of the trait being a true-breeding characteristic. Other recording entities or specialty organizations may recognize horses from multiple breeds, thus, for the purposes of this article, such animals are classified as a "type" rather than a "breed". The breeds and types listed here are those that already have a Wikipedia article. For a more extensive list, see the List of all horse breeds in DAD-IS. Heavy or draft horse breeds For additional information, see horse breed, horse breeding and the individual articles listed below. -

Special Issue Horse Genetics DIRECTOR’S Message
CENTER FOR EQUINE HEALTH SCHOOL OF VETERINARY MEDICINE • UNIVERSITY OF CALIFORNIA, DAVIS SUMMER 2020 Special Issue Horse Genetics DIRECTOR’S Message s an equine genetics researcher, I am particularly excited to share A this special issue of the Horse Report with you. Inside, you will find a roadmap to many of the currently available equine genetic tests, including the AQHA “five-panel” test, and more. The equine genome sequence was published in 2009, the result of a years- long collaborative effort by the international equine research community. This resource drastically changed how researchers approach equine genetics and accelerated the rate of discovery. Increased availability and affordability allowed the application of advanced molecular tools to equine diseases and traits. As a result, genetic tests are available in a variety of breeds. Most available tests are for simple, Mendelian diseases and traits – those caused by a single gene or locus. Complex diseases and traits likely involve more than one gene and may be influenced by environmental effects. The 2018 release of a new equine genome sequence assembly, coupled with cost reductions that make whole-genome sequencing possible for large numbers of horses, are enabling research in these areas. As an equine geneticist and veterinarian, I am especially interested in applying whole genome sequencing and advanced diagnostic tools to equine precision medicine. This highly individualized approach will focus on early detection and prevention of disease, taking into account both genetic information and environmental factors. The idea is to target individuals based on their clinical condition as well as their unique body chemistry and genetics. -

Cob Or Chimera?
Cob or Chimera? UK Expat Sarah Redstone Lee explains why she believes the Cob Normand to be the most versatile cob of them all nce we had decided to take the The origins of the Normandy Cob can be To this day, the Chateau of Versailles is still plunge, sell up, pack up and traced back to Great Britain, the first ‘Norman’ surrounded by carriage driving Normandy make the move to Normandy; horse, to arrive on our shores was during the Cobs in homage to his passion for the breed. code named Operation transportation of a herd of around 2- 3,000, in The National Stud and home of the Overseas ( as but a small preparations for the battle of Hastings of 1066. Normandy Cob was founded by Napoléon in homage to operation Overlord in Normandy, Much of the success of the invasion depended 1806. By 1840, some of the cobs were crossed Owhich formed part of the D-Day landings), it on the horses that were bred for the endurance with King Henry the VIII’s favourite breed, was not long before my husband and I were required in order to carry the weight of the now extinct Norfolk trotter. This further enjoying la belle vie Francaise. We have been armoured suits, weapons and chain mail, yet the enhanced the look of elegance combined here for almost 10 years now, and, aside from superior among them also possessed great speed. with speed. The standard was set and the benefitting from the usual attractions that The Normans, are even to this day well Normandy Cob was then divided in to two France has to offer, that needs no form of known for their breeding of the Percheron groups; heavier horses for work, and lighter introduction, I have been very privileged to and the French Trotter. -

Electronic Supplementary Material - Appendices
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

2008 Eastern National 4-H Horse Bowl Round 7
2008 Eastern National 4-H Horse Bowl Round 7 One-On-One 1. C1 Q. What is bradycardia? A. An abnormally slow heart rate S. DET p. 37 440/3 2. C2 Q. The following horses are part of the history of what breed… Bourbon King, Wing Commander and Denmark? A. American Saddlebred horse S. HIH 162-2 200/3 3. C3 Q. If you are “fuzztail running”, what are you doing? A. Herding and catching wild horses S. DET p. 116 920/4 4. C4 Q. In the zoological classification what family includes the horse? A. Equidae S. Evans p.13 100/2 1 2008 Eastern National 4-H Horse Bowl Round 7 5. C1 Q. What is the only function of the vitamin D in the horse’s body? A. Maintaining plasma calcium levels – by regulating calcium absorption S. Lewis p. 48, Evans p. 221 740/3 6. C2 Q. What is the pangaré effect on color? A. Results in light areas of color on the muzzle, over the eyes, on the flanks and inside the legs S. Evans p.481 310/3 7. C3 Q. In the history of Spain during the reign of Queen Isabella, what current breed of horses was known as Golden Isabellas? A. Palomino S. Evans p.50 200/3 8. C4 Q. What sex-linked disease results in a deficiency of clotting factor VIII? A. Hemophilia A (also accept Hemophilia) S. Evans p.511 550/3 2 2008 Eastern National 4-H Horse Bowl Round 7 9. C1 Q. In show jumping, a “combination obstacle” consists of two or more separate jumps that are numbered and lettered. -

All Senior Questions Question Answer Source
All Senior Questions Question Answer Source Where in the digestive tract are amino acids Large intestine. HIH 710 synthesized? What unsoundness is most noticeable when Stringhalt. Ensminger, 530 backing the horse? Describe the ideal angles of the horse's front feet Front feet: 45 - 50 degrees. Beeman, 8 and hind feet. Rear feet: 50 - 60 degrees. An excessive reaction of the skin to sunlight is Photosensitivity. Veterinary Medicine, called what? 591 What term is used to describe a hoof wall angle Club foot. Curtis, 45 of 65 degrees or more? The American Paint Horse Association is devoted Paints, Quarter Horses, and Thoroughbreds. HBM strictly to stock horses and bases its registry on the blood of what 3 breeds? What do the letters CF stand for? Crude Fiber. Ensminger, 550 The common digital artery supplies blood to what Phalanges and foot. HBM parts of the horse? What is the interdental space? The gum space between the incisors and the HBM molars. Thursday, January 03, 1980 Page 1 of 95 University of Kentucky, College of Agriculture,Cooperative Extenison Service All Senior Questions Question Answer Source What color horses are more commonly prone to Gray horses. Veterinary Medicine, melanomas? 307 Most of the nutrients are found in what part of the Leaves. HBM forage plant? Excessive granulation tissue rising out of and Proud flesh. Ensminger, 527 above the edges of a wound is called what? Explain the functional difference of arteries and Arteries carry blood away from the heart to the Evans, Borton et all, veins in the horse's body. body tissues. -

Lipizzan Laurels United States Lipizzan Federation©
Lipizzan Laurels United States Lipizzan Federation© Awards Program The United States Lipizzan Federation (USLF) recognizes Lipizzans/XL Lipizzans competing in events against all breeds with two special award programs—the USLF Lipizzan/XL Lipizzan Laurels Award and the USLF Lipizzan/XL Lipizzan Star Award. Award participants automatically are entered in both programs when they submit results. The USLF Lipizzan Laurels presents a Lipizzan Laurels Award for outstanding performance by a Lipizzan/XL Lipizzan horse in several sections within the program’s ten major disciplines. Junior Exhibitor Awards are also presented in all ten disciplines. Lipizzan Laurels awards are tabulated on an annual basis during the competition year, which runs from November 1st of the previous year to October 31st of the current year. The Lipizzan/XL Lipizzan Star Award is a lifetime award presented to each Lipizzan/XL Lipizzan meeting Star requirements. Lipizzan/XL Lipizzan horses may take as many years as needed to earn Star points. The owner of the achieving horse will receive an official bronze, silver, gold, or platinum Star when they have accumulated the necessary points in their discipline. Stars are presented in ten disciplines: Show, Competitive Trail, Endurance, Dressage, Eventing, Working Western, Carriage Pleasure, Combined Driving, Western Dressage, and Working Equitation. Horses who earn five of the ten bronze Stars are presented with the USLF Lipizzan/XL Lipizzan Sport Horse Award. 1 GENERAL GUIDELINES To be eligible for these awards, horses must be registered with the USLF at the time scores are earned. Both the owner and all riders or drivers of the horse must be current USLF members at the time scores are earned. -

Quantitative Genetic Analysis of Melanoma and Grey Level in Lipizzan Horses
7th World Congress on Genetics Applied to Livestock Production, August 19-23, 2002, Montpellier, France QUANTITATIVE GENETIC ANALYSIS OF MELANOMA AND GREY LEVEL IN LIPIZZAN HORSES I. Curik1, M. Seltenhammer2 and J. Sölkner3 1Animal Science Department, Faculty of Agriculture, University of Zagreb, Croatia 2Clinic of Surgery and Ophthalmology, University of Veterinary Medicine Vienna, Austria 3Department of Livestock Science, University of Agricultural Sciences Vienna, Austria INTRODUCTION Changes of coat colour in which the "dark (non-grey)" colour present in foals is progressively replaced by grey is a known phenomenon in horses. A similar process is present in humans. The grey coat colour is inherited as a dominant trait and is the characteristic, although not exclusive, colour for some horse breeds (Bowling, 2000). The Lipizzan horse, originally bred for show and parade at the Imperial Court in Vienna, is among those breeds. Unfortunately, melanomas (skin tumours) are more prevalent in grey than in non-grey horses (e.g. Seltenhammer, 2000). The causative relationship for this positive association as well as the molecular basis for both traits (melanoma and grey level) are not known (Rieder, 1999 ; Seltenhammer, 2000). The inheritance of coat colour in horses has been always studied from a qualitative view (Sponenberg, 1996 ; Bowling, 2000). In the present study we quantified the grey level (shade) and estimated the proportion of additive genetic component (heritability in the narrow sense) of this trait. Further, we estimated the genetic relationship between melanoma stages and grey level as well as the additive inheritance of melanoma stages. MATERIAL AND METHODS Horses. Data for this study was collected from 351 grey Lipizzan horses of four national studs (Djakovo – Croatia ; 64 horses, Piber – Austria ; 160 horses, Szilvesvarad – Hungary ; 67 horses and Topol'cianky – Slovakia ; 60 horses). -
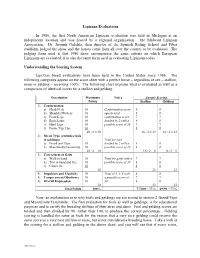
2-Evaluation Summary (H0933573
Lipizzan Evaluations In 1986, the first North American Lipizzan evaluation was held in Michigan at an independent location and was hosted by a regional organization – the Midwest Lipizzan Association. Dr. Jaromir Oulehla, then director of the Spanish Riding School and Piber studfarm, judged the show and the horses came from all over the country to be evaluated. The judging form used at that 1986 show encompasses the same criteria on which European Lipizzans are evaluated; it is also the same form used in evaluating Lipizzans today. Understanding the Scoring System Lipizzan breed evaluations have been held in the United States since 1986. The following categories appear on the score sheet with a perfect horse – regardless of sex – stallion, mare or gelding – receiving 100%. The following chart explains what is evaluated as well as a comparison of identical scores for a stallion and gelding. Description Maximum Notes Sample Scoring Points Stallion Gelding 1) Conformation a) Head/Neck 10 Conformation score 8 8 b) Shoulder/Withers 10 equals total 8 8 c) Front Legs 10 confirmation score 7 7 d) Back/Loins 10 divided by 2 with a 8 8 e) Hind Legs 10 possible score of 30 7 7 f) Frame/Top Line 10 8 8 60 / 2 = 30 46 / 2 = 23 46 / 2 = 23 2) Breed Type (excludes foals & geldings) Total for type a) Breed and Type 10 divided by 2 with a 8 0 b) Masculinity/Femininity 10 possible score of 10 8 0 20 / 2 = 10 16 / 2 = 8 0 / 2 = 0 3) Correctness of Gaits a) Walk in hand 10 Total for gaits with a 7 7 b) Trot in hand and free 10 possible score of 30 8 8 c) Canter free 10 8 8 30 23 23 4) Impulsion and Elasticity 10 Total of 4, 5, 6 with 8 8 5) Temperament/Obedience 10 a possible score of 7 7 6) Overall Impression 10 30 8 8 30 23 23 Total Points 100% 77/100 = 77% 69/90 = 77% Now, an explanation as to why foals and geldings are not scored in section 2 Breed/Type and Masculinity/Femininity. -

Basic Horse Genetics
ALABAMA A&M AND AUBURN UNIVERSITIES Basic Horse Genetics ANR-1420 nderstanding the basic principles of genetics and Ugene-selection methods is essential for people in the horse-breeding business and is also beneficial to any horse owner when it comes to making decisions about a horse purchase, suitability, and utilization. Before getting into the basics of horse-breeding deci- sions, however, it is important that breeders under- stand the following terms. Chromosome - a rod-like body found in the cell nucleus that contains the genes. Chromosomes occur in pairs in all cells, with the exception of the sex cells (sperm and egg). Horses have 32 pairs of chromo- somes, and donkeys have 31 pairs. Gene - a small segment of chromosome (DNA) that contains the genetic code. Genes occur in pairs, one Quantitative traits - traits that show a continuous on each chromosome of a pair. range of phenotypic variation. Quantitative traits Alleles - the alternative states of a particular gene. The usually are controlled by more than one gene pair gene located at a fixed position on a chromosome will and are heavily influenced by environmental factors, contain a particular gene or one of its alleles. Multiple such as track condition, trainer expertise, and nutrition. alleles are possible. Because of these conditions, quantitative traits cannot be classified into distinct categories. Often, the impor- Genotype - the genetic makeup of an individual. With tant economic traits of livestock are quantitative—for alleles A and a, three possible genotypes are AA, Aa, example, cannon circumference and racing speed. and aa. Not all of these pairs of alleles will result in the same phenotype because pairs may have different Heritability - the portion of the total phenotypic modes of action. -

Arabian Coat Color Patterns
Arabian Coat Color Patterns Copyright 2011 Brenda Wahler In the Arabian breed, there are three unusual coat colors or patterns that occur in some purebred horses. The first is sabino, the only white spotting pattern seen in purebred Arabians, characterized by bold white face and leg markings, and, in some cases, body spotting. The second pattern is rabicano, a roan-like intermixture of white and dark hairs. Both sabino and rabicano horses are often registered by their base coat color, with white patterns noted as markings, but some extensively marked individuals have been registered as “roan,” even though true roan is a separate coat color. The third unusual coat color is dominant white, a mutation characterized by a predominantly white hair coat and pink skin, present at birth. All Arabians in the United States currently known to be dominant white trace to a single stallion, foaled in 1996, verified to be the offspring of his registered Arabian parents, both of whom were solid-colored. It is difficult to know how many Arabians have these unusual colors as they are often not searchable in registration records. For many years, Arabians with dominant white, body spots, or simply “too much white” were discouraged from registration, and white body markings were penalized in halter classes. The exclusion of boldly-marked “cropout” horses was also common in other registries, leading to the formation of a number of color breed associations. However, when parentage verification became possible, horses born with “too much” white could be confirmed as the offspring of their stated parents, and breed registries generally relaxed their rules or policies that previously excluded such animals.