GENETIC DIVERSITY ASSESSMENT of an INDIGENOUS HORSE POPULATION of GREECE Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Horse Breeds 1 List of Horse Breeds
List of horse breeds 1 List of horse breeds This page is a list of horse and pony breeds, and also includes terms used to describe types of horse that are not breeds but are commonly mistaken for breeds. While there is no scientifically accepted definition of the term "breed,"[1] a breed is defined generally as having distinct true-breeding characteristics over a number of generations; its members may be called "purebred". In most cases, bloodlines of horse breeds are recorded with a breed registry. However, in horses, the concept is somewhat flexible, as open stud books are created for developing horse breeds that are not yet fully true-breeding. Registries also are considered the authority as to whether a given breed is listed as Light or saddle horse breeds a "horse" or a "pony". There are also a number of "color breed", sport horse, and gaited horse registries for horses with various phenotypes or other traits, which admit any animal fitting a given set of physical characteristics, even if there is little or no evidence of the trait being a true-breeding characteristic. Other recording entities or specialty organizations may recognize horses from multiple breeds, thus, for the purposes of this article, such animals are classified as a "type" rather than a "breed". The breeds and types listed here are those that already have a Wikipedia article. For a more extensive list, see the List of all horse breeds in DAD-IS. Heavy or draft horse breeds For additional information, see horse breed, horse breeding and the individual articles listed below. -

Electronic Supplementary Material - Appendices
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

Lipizzan Laurels United States Lipizzan Federation©
Lipizzan Laurels United States Lipizzan Federation© Awards Program The United States Lipizzan Federation (USLF) recognizes Lipizzans/XL Lipizzans competing in events against all breeds with two special award programs—the USLF Lipizzan/XL Lipizzan Laurels Award and the USLF Lipizzan/XL Lipizzan Star Award. Award participants automatically are entered in both programs when they submit results. The USLF Lipizzan Laurels presents a Lipizzan Laurels Award for outstanding performance by a Lipizzan/XL Lipizzan horse in several sections within the program’s ten major disciplines. Junior Exhibitor Awards are also presented in all ten disciplines. Lipizzan Laurels awards are tabulated on an annual basis during the competition year, which runs from November 1st of the previous year to October 31st of the current year. The Lipizzan/XL Lipizzan Star Award is a lifetime award presented to each Lipizzan/XL Lipizzan meeting Star requirements. Lipizzan/XL Lipizzan horses may take as many years as needed to earn Star points. The owner of the achieving horse will receive an official bronze, silver, gold, or platinum Star when they have accumulated the necessary points in their discipline. Stars are presented in ten disciplines: Show, Competitive Trail, Endurance, Dressage, Eventing, Working Western, Carriage Pleasure, Combined Driving, Western Dressage, and Working Equitation. Horses who earn five of the ten bronze Stars are presented with the USLF Lipizzan/XL Lipizzan Sport Horse Award. 1 GENERAL GUIDELINES To be eligible for these awards, horses must be registered with the USLF at the time scores are earned. Both the owner and all riders or drivers of the horse must be current USLF members at the time scores are earned. -

Discriminant Canonical Analysis of the Contribution of Spanish and Arabian Purebred Horses to the Genetic Diversity and Population Structure of Hispano-Arabian Horses
UC Davis UC Davis Previously Published Works Title Discriminant Canonical Analysis of the Contribution of Spanish and Arabian Purebred Horses to the Genetic Diversity and Population Structure of Hispano-Arabian Horses. Permalink https://escholarship.org/uc/item/8w77w522 Journal Animals : an open access journal from MDPI, 11(2) ISSN 2076-2615 Authors Marín Navas, Carmen Delgado Bermejo, Juan Vicente McLean, Amy Katherine et al. Publication Date 2021-01-21 DOI 10.3390/ani11020269 Peer reviewed eScholarship.org Powered by the California Digital Library University of California animals Article Discriminant Canonical Analysis of the Contribution of Spanish and Arabian Purebred Horses to the Genetic Diversity and Population Structure of Hispano-Arabian Horses Carmen Marín Navas 1 , Juan Vicente Delgado Bermejo 1 , Amy Katherine McLean 2 , José Manuel León Jurado 3, Antonio Rodriguez de la Borbolla y Ruiberriz de Torres 4 and Francisco Javier Navas González 1,* 1 Department of Genetics, Faculty of Veterinary Sciences, University of Córdoba, 14071 Córdoba, Spain; [email protected] (C.M.N.); [email protected] (J.V.D.B.) 2 Department of Animal Science, University of California Davis, Davis, CA 95617, USA; [email protected] 3 Centro Agropecuario Provincial de Córdoba, Diputación Provincial de Córdoba, 14071 Córdoba, Spain; [email protected] 4 Unión Española de Ganaderos de Pura Raza Hispano-Árabe, 41001 Sevilla, Spain; [email protected] * Correspondence: [email protected]; Tel.: +34-957-21-87-06 Simple Summary: The demographic and genetic diversity structure and the contributions of Spanish (PRE) and Arabian Purebred (PRá) horses to the process of conformation of the Hispano-Arabian Citation: Marín Navas, C.; Delgado (Há) horse breed were evaluated. -

Quantitative Genetic Analysis of Melanoma and Grey Level in Lipizzan Horses
7th World Congress on Genetics Applied to Livestock Production, August 19-23, 2002, Montpellier, France QUANTITATIVE GENETIC ANALYSIS OF MELANOMA AND GREY LEVEL IN LIPIZZAN HORSES I. Curik1, M. Seltenhammer2 and J. Sölkner3 1Animal Science Department, Faculty of Agriculture, University of Zagreb, Croatia 2Clinic of Surgery and Ophthalmology, University of Veterinary Medicine Vienna, Austria 3Department of Livestock Science, University of Agricultural Sciences Vienna, Austria INTRODUCTION Changes of coat colour in which the "dark (non-grey)" colour present in foals is progressively replaced by grey is a known phenomenon in horses. A similar process is present in humans. The grey coat colour is inherited as a dominant trait and is the characteristic, although not exclusive, colour for some horse breeds (Bowling, 2000). The Lipizzan horse, originally bred for show and parade at the Imperial Court in Vienna, is among those breeds. Unfortunately, melanomas (skin tumours) are more prevalent in grey than in non-grey horses (e.g. Seltenhammer, 2000). The causative relationship for this positive association as well as the molecular basis for both traits (melanoma and grey level) are not known (Rieder, 1999 ; Seltenhammer, 2000). The inheritance of coat colour in horses has been always studied from a qualitative view (Sponenberg, 1996 ; Bowling, 2000). In the present study we quantified the grey level (shade) and estimated the proportion of additive genetic component (heritability in the narrow sense) of this trait. Further, we estimated the genetic relationship between melanoma stages and grey level as well as the additive inheritance of melanoma stages. MATERIAL AND METHODS Horses. Data for this study was collected from 351 grey Lipizzan horses of four national studs (Djakovo – Croatia ; 64 horses, Piber – Austria ; 160 horses, Szilvesvarad – Hungary ; 67 horses and Topol'cianky – Slovakia ; 60 horses). -

Crossbreeding the Andalusian Horse in Short
Crossbreeding the Andalusian Horse By Sarah Gately-Wilson The Andalusian horse is growing in popularity and its future is full of endless possibilities. The purebred Andalusian is highly versatile and capable of being successful in any discipline; however, with just over 10,000 registered purebreds in the U.S. these horses are still very rare and not easy to acquire. To fill the growing demand for the qualities the Andalusians possess many breeders are turning to crossbreeding. Some of the crosses have been bred long enough to establish breeds in their own right and some are just getting started. A few acknowledged crosses include the Iberian Warmblood, the Azteca (AQHA), the Spanish-Norman (Percheron), the Warlander (Friesian), and the Hispano-Arabe. When looking for an Andalusian to breed, whether to another Andalusian or to an outside breed, you should look for one with a good-looking head set on an arching neck, a broad forehead, well-placed ears, and almond shaped eyes that are alive and kind. The Andalusian should have an abundant tail, set low and hung tightly against the body; the mane as well should be thick. It should have well-defined withers preceding a short back and broad strong hindquarters. When Spain claimed the New World, the Spanish horse was there to help. On his second voyage in 1493, Christopher Columbus brought the Andalusian horse to the Americas. Every subsequent expedition also contained Andalusians in its Cargo. Breeding farms were established in the Caribbean to provide mounts for the Conquistadors as they explored and settled the New World. -
2-Evaluation Summary (H0933573
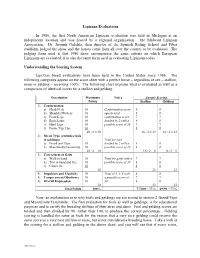
Lipizzan Evaluations In 1986, the first North American Lipizzan evaluation was held in Michigan at an independent location and was hosted by a regional organization – the Midwest Lipizzan Association. Dr. Jaromir Oulehla, then director of the Spanish Riding School and Piber studfarm, judged the show and the horses came from all over the country to be evaluated. The judging form used at that 1986 show encompasses the same criteria on which European Lipizzans are evaluated; it is also the same form used in evaluating Lipizzans today. Understanding the Scoring System Lipizzan breed evaluations have been held in the United States since 1986. The following categories appear on the score sheet with a perfect horse – regardless of sex – stallion, mare or gelding – receiving 100%. The following chart explains what is evaluated as well as a comparison of identical scores for a stallion and gelding. Description Maximum Notes Sample Scoring Points Stallion Gelding 1) Conformation a) Head/Neck 10 Conformation score 8 8 b) Shoulder/Withers 10 equals total 8 8 c) Front Legs 10 confirmation score 7 7 d) Back/Loins 10 divided by 2 with a 8 8 e) Hind Legs 10 possible score of 30 7 7 f) Frame/Top Line 10 8 8 60 / 2 = 30 46 / 2 = 23 46 / 2 = 23 2) Breed Type (excludes foals & geldings) Total for type a) Breed and Type 10 divided by 2 with a 8 0 b) Masculinity/Femininity 10 possible score of 10 8 0 20 / 2 = 10 16 / 2 = 8 0 / 2 = 0 3) Correctness of Gaits a) Walk in hand 10 Total for gaits with a 7 7 b) Trot in hand and free 10 possible score of 30 8 8 c) Canter free 10 8 8 30 23 23 4) Impulsion and Elasticity 10 Total of 4, 5, 6 with 8 8 5) Temperament/Obedience 10 a possible score of 7 7 6) Overall Impression 10 30 8 8 30 23 23 Total Points 100% 77/100 = 77% 69/90 = 77% Now, an explanation as to why foals and geldings are not scored in section 2 Breed/Type and Masculinity/Femininity. -

Usefulness of the 17-Plex Str Kit for Bosnian Mountain Horse Genotyping
UDC 575. https://doi.org/10.2298/GENSR1902619R Original scientific paper USEFULNESS OF THE 17-PLEX STR KIT FOR BOSNIAN MOUNTAIN HORSE GENOTYPING Dunja RUKAVINA1*, Amir ZAHIROVIĆ2, Ćazim CRNKIĆ3, Mirela MAČKIĆ-ĐUROVIĆ4, Adaleta DURMIĆ-PAŠIĆ5, Belma KALAMUJIĆ STROIL5, Naris POJSKIĆ5 1*University of Sarajevo-Veterinary Faculty, Department of Biology, Sarajevo, B&H 2University of Sarajevo-Veterinary Faculty, Department of Internal Diseases, Sarajevo, B&H 3University of Sarajevo-Veterinary Faculty, Department of Animal Nutrition, Sarajevo, B&H 4University of Sarajevo-Faculty of Medicine, Center for Genetic, Sarajevo, B&H 5University of Sarajevo-Institute for Genetic Engineering and Biotechnology, Sarajevo, B&H Rukavina D., A. Zahirović, Ć. Crnkić, M. Mačkić-Đurović, A. Durmić-Pašić, B. Kalamujić Stroil, N. Pojskić (2019): Usefulness of the 17-plex STR kit for Bosnian mountain horse genotyping.- Genetika, Vol 51, No.2, 619-627. In the present study modern technology of DNA extraction and automatic genotyping was applied in Bosnian and Herzegovinian autochthonous horse breed by using 17-Plex horse genotyping kit. The study was aimed at investigating usefulness of the 17-plex STR Kit for Bosnian mountain horse genotyping and establishing highly useful microsatellite markers system for genetic diversity studies in Bosnian mountain horse breed. Genomic DNA was extracted from whole blood collected from 22 unrelated Bosnian mountain horse specimens. A total of 95 alleles were detected. Average number of detected alleles per locus was 5.588, varying from 3 (HTG7) to 10 (ASB17). Average effective number of alleles was 3.603, fluctuating from 1.789 (HMS7) to 5.728 (HMS2). The observed heterozygosity ranged from 0.136 (HMS3) to 0.909 (ASB2) with a mean of 0.631. -

Complaint Report
EXHIBIT A ARKANSAS LIVESTOCK & POULTRY COMMISSION #1 NATURAL RESOURCES DR. LITTLE ROCK, AR 72205 501-907-2400 Complaint Report Type of Complaint Received By Date Assigned To COMPLAINANT PREMISES VISITED/SUSPECTED VIOLATOR Name Name Address Address City City Phone Phone Inspector/Investigator's Findings: Signed Date Return to Heath Harris, Field Supervisor DP-7/DP-46 SPECIAL MATERIALS & MARKETPLACE SAMPLE REPORT ARKANSAS STATE PLANT BOARD Pesticide Division #1 Natural Resources Drive Little Rock, Arkansas 72205 Insp. # Case # Lab # DATE: Sampled: Received: Reported: Sampled At Address GPS Coordinates: N W This block to be used for Marketplace Samples only Manufacturer Address City/State/Zip Brand Name: EPA Reg. #: EPA Est. #: Lot #: Container Type: # on Hand Wt./Size #Sampled Circle appropriate description: [Non-Slurry Liquid] [Slurry Liquid] [Dust] [Granular] [Other] Other Sample Soil Vegetation (describe) Description: (Place check in Water Clothing (describe) appropriate square) Use Dilution Other (describe) Formulation Dilution Rate as mixed Analysis Requested: (Use common pesticide name) Guarantee in Tank (if use dilution) Chain of Custody Date Received by (Received for Lab) Inspector Name Inspector (Print) Signature Check box if Dealer desires copy of completed analysis 9 ARKANSAS LIVESTOCK AND POULTRY COMMISSION #1 Natural Resources Drive Little Rock, Arkansas 72205 (501) 225-1598 REPORT ON FLEA MARKETS OR SALES CHECKED Poultry to be tested for pullorum typhoid are: exotic chickens, upland birds (chickens, pheasants, pea fowl, and backyard chickens). Must be identified with a leg band, wing band, or tattoo. Exemptions are those from a certified free NPIP flock or 90-day certificate test for pullorum typhoid. Water fowl need not test for pullorum typhoid unless they originate from out of state. -

Pedigree Analysis in the Andalusian Horse: Population Structure, Genetic Variability and Influence of the Carthusian Strain
Livestock Production Science 95 (2005) 57–66 www.elsevier.com/locate/livprodsci Pedigree analysis in the Andalusian horse: population structure, genetic variability and influence of the Carthusian strain M. Valeraa,*, A. Molinab, J.P. Gutie´rrezc,J.Go´mezb, F. Goyached aDepartamento de Ciencias Agroforestales, Escuela de Ingenierı´a Te´cnica Agrı´cola, Universidad de Sevilla, Ctra. Utrera km 1, 41013-Sevilla, Spain bDepartamento de Gene´tica de la Universidad de Co´rdoba, Ctra. Madrid-Co´rdoba, km. 373a., 14071-Co´rdoba, Spain cDepartamento de Produccio´n Animal, Facultad de Veterinaria, Avda. Puerta de Hierro s/n, E-28040-Madrid, Spain dSERIDA-Somio´, C/Camino de los Claveles 604, E-33203 Gijo´n (Asturias), Spain Received 27 July 2004; received in revised form 26 November 2004; accepted 9 December 2004 Abstract The studbook of the Andalusian horse comprising a total of 75,389 individuals (6318 of them classified as Carthusians) was analysed in order to ascertain the genetic history of the breed and, specially, to evaluate its genetic variability and the influence of the Carthusian strain in the breed. Although there is no possible way to identify studs acting as Nucleus in the breed, there is a high concentration of genes and individuals’ origin. The effective number of studs producing grandfathers was of 10.6. The equivalent number of founders was 1948.5 individuals (370.5 Carthusians). The effective number of founders was 39.6 and the effective number of ancestors 27. Only 6 ancestors were necessary to explain 50% of the genetic variability of the breed. The average values of inbreeding and average relatedness for the whole Andalusian horse population were, respectively, 8.48% and 12.25%. -

Andalusian/Lusitano Breed Show
n R t e r e g s i o a n E A b n u d l a C l The Journal of ERAHC The Eastern Region Andalusian Horse Club u s e — si a o r n H August 2013 The 2013 New England Classic Horse Shows The NE Classic shows were exciting, enjoyable, and successful. There were 63 horses entered in the dressage show with classes ranging from training level through Grand Prix. There were 33 horses entered in the breed show covering a full spectrum of classes, including the inaugural Baroque Eques- trian Games rail classes. Some of the more popular classes were Best Move- ment and Dressage Sport Horse in Hand (for both A/L and HA), as well as Mounted Trail, English Pleasure Hunt Seat, and Working Equitation. The weather was a factor on Friday, but the staff and exhibitors managed in spite of the extreme 100-degree heat. The club provided buckets of ice water and towels, plus cups of cool water for the dressage exhibitors as they left the arenas. The weather did cool down for the breed show on Saturday and even more on Sunday. The Saturday evening Iberian Extravaganza was well choreographed and very entertaining. Patricia Norcia spent countless hours organizing the ex- hibitions and conducting rehearsals for our show. She also conducted fund- raisers to offset the cost of these exhibitions. We thank Patricia for the many years she has provided her expertise and dedication to the ERAHC NE Clas- sic. We also wish to extend thanks to our corporate sponsors who generously donated financial support to our show. -

The Myostatin Gene: an Overview of Mechanisms of Action and Its Relevance to Livestock Animals
The Myostatin gene: an overview of mechanisms of action and its relevance to livestock animals Article Accepted Version Aiello, D., Patel, K. and Lasagna, E. (2018) The Myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Animal Genetics, 49 (6). pp. 505-519. ISSN 1365-2052 doi: https://doi.org/10.1111/age.12696 Available at http://centaur.reading.ac.uk/77388/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . To link to this article DOI: http://dx.doi.org/10.1111/age.12696 Publisher: Wiley All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online 1 Review: The Myostatin gene: an overview of mechanisms of action and its 2 relevance to livestock animals 3 D. Aiello 1, K. Patel 2 and E. Lasagna 1 4 5 1 Dipartimento di Scienze Agrarie, Alimentari e Ambientali, Università degli Studi di 6 Perugia, Borgo XX Giugno 74, 06121, Perugia, Italy 7 2 School of Biological Sciences, University of Reading, Berkshire, RG6 6UB, United 8 Kingdom 9 10 Corresponding author: Emiliano Lasagna. Fax: +39 075 5857122. Tel: +39 075 11 5857102. E-mail address: [email protected] 12 13 1 14 15 Summary 16 Myostatin, also known as Growth Differentiation Factor 8, a member of the 17 Transforming Growth Factor-beta (TGF-β) super-family is a negative regulator of 18 muscle development.