Consequences of the Domestication of Man's Best Friend, The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Maladaptation in Feral and Domesticated Animals
Nova Southeastern University NSUWorks Biology Faculty Articles Department of Biological Sciences 8-2019 Maladaptation in Feral and Domesticated Animals Eben Gering Michigan State University, [email protected] Darren Incorvaia Michigan State University R. Henriksen Linkoping University- Sweden Dominic Wright Linkoping University- Sweden Follow this and additional works at: https://nsuworks.nova.edu/cnso_bio_facarticles Part of the Biology Commons NSUWorks Citation Gering, Eben; Darren Incorvaia; R. Henriksen; and Dominic Wright. 2019. "Maladaptation in Feral and Domesticated Animals." Evolutionary Applications 12, (7): 1274-1286. doi:10.1111/eva.12784. This Article is brought to you for free and open access by the Department of Biological Sciences at NSUWorks. It has been accepted for inclusion in Biology Faculty Articles by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Received: 17 October 2018 | Revised: 10 January 2019 | Accepted: 7 February 2019 DOI: 10.1111/eva.12784 SPECIAL ISSUE REVIEW AND SYNTHESES Maladaptation in feral and domesticated animals Eben Gering1 | Darren Incorvaia1 | Rie Henriksen2 | Dominic Wright2 | Thomas Getty1 1Department of Integrative Biology and Ecology, Evolutionary Biology, and Behavior Abstract Program, Michigan State University, East Selection regimes and population structures can be powerfully changed by domesti‐ Lansing, Michigan cation and feralization, and these changes can modulate animal fitness in both cap‐ 2IIFM Biology and AVIAN Behavioural Genomics and Physiology Group, Linköping tive and natural environments. In this review, we synthesize recent studies of these University, Sweden two processes and consider their impacts on organismal and population fitness. Correspondence Domestication and feralization offer multiple windows into the forms and mecha‐ Eben Gering, Department of Integrative nisms of maladaptation. -

The Evolution of Animal Domestication
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/266740619 The Evolution of Animal Domestication Article in Annual Review of Ecology Evolution and Systematics · October 2014 DOI: 10.1146/annurev-ecolsys-120213-091620 CITATIONS READS 179 3,162 2 authors: Greger Larson Dorian Q Fuller University of Oxford University College London 196 PUBLICATIONS 6,523 CITATIONS 322 PUBLICATIONS 12,021 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Dog Domestication View project Rwandan Archaeology View project All content following this page was uploaded by Dorian Q Fuller on 12 October 2014. The user has requested enhancement of the downloaded file. ES45CH06-Larson ARI 16 September 2014 11:18 V I E E W R S I E N C N A D V A The Evolution of Animal Domestication Greger Larson1 and Dorian Q. Fuller2 1Durham Evolution and Ancient DNA, Department of Archaeology, Durham University, Durham, DH1 3LE, United Kingdom; email: [email protected] 2Institute of Archaeology, University College London, London WC1H 0PY, United Kingdom Annu. Rev. Ecol. Evol. Syst. 2014. 66:115–36 Keywords The Annual Review of Ecology, Evolution, and archaeology, genetics, livestock, introgression, selection, agriculture Systematics is online at ecolsys.annualreviews.org This article’s doi: Abstract 10.1146/annurev-ecolsys-120213-091620 The domestication of plants and animals over the past 11,500 years has Copyright c 2014 by Annual Reviews. had a significant effect not just on the domesticated taxa but also on human All rights reserved evolution and on the biosphere as a whole. -

And Ford, I; Ford, '953) on the Other Hand Have Put Forward a View Intermediate Between the Extreme Ones of Darwin on the One Hand and Goldschmidt on the Other
THE EVOLUTION OF MIMICRY IN THE BUTTERFLY PAPILIO DARDANUS C. A. CLARKE and P. M. SHEPPARD Departments of Medicine and Zoology, University of Liverpool Received23.V.59 1.INTRODUCTION WHENBatesputforward the mimicry hypothesis which bears his name, Darwin (1872), although accepting it, had some difficulty in explaining the evolution of the mimetic resemblance of several distinct species to one distasteful model by a series of small changes, a require- ment of his general theory of evolution. He said "it is necessary to suppose in some cases that ancient members belonging to several distinct groups, before they had diverged to their present extent, accidentally resembled a member of another and protected group in a sufficient degree to afford some slight protection; this having given the basis for the subsequent acquisition of the most perfect resemb- lance ". Punnett (1915) realised that the difficulty is even more acute when one is dealing with a polymorphic species whose forms mimic very distantly related models. Knowing that, in those butterflies which had been investigated genetically, the forms differed by single allelomorphs he concluded that the mimicry did not evolve gradually and did not confer any advantage or disadvantage to the individual. He argued that an allelomorph arises at a single step by mutation and that therefore the mimicry also arises by chance at a single step. Goldschmidt (x) although not denying that mimicry confers some advantage to its possessors also maintained that the resemblance arises fully perfected by a single mutation of a gene distinct from that producing the colour pattern in the model, but producing a similar effect in the mimic. -

The History of Farm Foxes Undermines the Animal Domestication Syndrome, Trends in Ecology & Evolution (2019)
Please cite this article in press as: Lord et al., The History of Farm Foxes Undermines the Animal Domestication Syndrome, Trends in Ecology & Evolution (2019), https://doi.org/10.1016/j.tree.2019.10.011 Trends in Ecology & Evolution Opinion The History of Farm Foxes Undermines the Animal Domestication Syndrome Kathryn A. Lord,1,2 Greger Larson,3,@ Raymond P. Coppinger,4,6 and Elinor K. Karlsson1,2,5,@,* The Russian Farm-Fox Experiment is the best known experimental study in animal domestication. Highlights By subjecting a population of foxes to selection for tameness alone, Dimitry Belyaev generated The ‘domestication syndrome’ has foxes that possessed a suite of characteristics that mimicked those found across domesticated been a central focus of research species. This ‘domestication syndrome’ has been a central focus of research into the biological into the biological processes un- pathways modified during domestication. Here, we chart the origins of Belyaev’s foxes in derlying domestication. The eastern Canada and critically assess the appearance of domestication syndrome traits across an- Russian Farm-Fox Experiment was imal domesticates. Our results suggest that both the conclusions of the Farm-Fox Experiment the first to test whether there is a and the ubiquity of domestication syndrome have been overstated. To understand the process causal relationship between selec- tion for tameness and the domes- of domestication requires a more comprehensive approach focused on essential adaptations to tication syndrome. human-modified environments. Historical records and genetic The Origins of Domestication Syndrome analysis show that the foxes used in The domestication syndrome describes a suite of behavioral and morphological characteristics the Farm-Fox Experiment origi- consistently observed in domesticated populations. -

Microevolution and the Genetics of Populations Microevolution Refers to Varieties Within a Given Type
Chapter 8: Evolution Lesson 8.3: Microevolution and the Genetics of Populations Microevolution refers to varieties within a given type. Change happens within a group, but the descendant is clearly of the same type as the ancestor. This might better be called variation, or adaptation, but the changes are "horizontal" in effect, not "vertical." Such changes might be accomplished by "natural selection," in which a trait within the present variety is selected as the best for a given set of conditions, or accomplished by "artificial selection," such as when dog breeders produce a new breed of dog. Lesson Objectives ● Distinguish what is microevolution and how it affects changes in populations. ● Define gene pool, and explain how to calculate allele frequencies. ● State the Hardy-Weinberg theorem ● Identify the five forces of evolution. Vocabulary ● adaptive radiation ● gene pool ● migration ● allele frequency ● genetic drift ● mutation ● artificial selection ● Hardy-Weinberg theorem ● natural selection ● directional selection ● macroevolution ● population genetics ● disruptive selection ● microevolution ● stabilizing selection ● gene flow Introduction Darwin knew that heritable variations are needed for evolution to occur. However, he knew nothing about Mendel’s laws of genetics. Mendel’s laws were rediscovered in the early 1900s. Only then could scientists fully understand the process of evolution. Microevolution is how individual traits within a population change over time. In order for a population to change, some things must be assumed to be true. In other words, there must be some sort of process happening that causes microevolution. The five ways alleles within a population change over time are natural selection, migration (gene flow), mating, mutations, or genetic drift. -

UNIVERSITY of CALIFORNIA Los Angeles the Evolution of Animals Through Domestication and Other Human Relationships
UNIVERSITY OF CALIFORNIA Los Angeles The Evolution of Animals through Domestication and other Human Relationships: An Animal-Centered Approach A thesis submitted in partial satisfaction of the requirements for the degree Master of Arts in Anthropology by Steven Michael Ammerman 2017 ©Copyright by Steven Michael Ammerman 2017 ABSTRACT OF THE THESIS The Evolution of Animals through Domestication and other Human Relationships: An Animal-Centered Approach by Steven Michael Ammerman Master of Arts in Anthropology University of California, Los Angeles, 2017 Professor Monica L. Smith, Chair As a component of the environment themselves, humans maintain a mutualistic modifying process with that environment. The interaction between humans and animals has led to different categories of relationships—commensal animals, tame animals, domesticated animals, and feral animals. In the study of human-animal interactions, the foremost consideration is how the niche construction of humans affects the evolutionary trajectory of other animals, but other animals also construct niches within this human-impacted environment to enhance their survival. The concept of niche construction provides a useful way of thinking about how humans and animals interact in the environment because this concept considers physical changes, behavioral changes, and cultural changes. In an animal-centered approach, consideration of how the traits and actions of animals play an active role in allowing and encouraging their ongoing relationships with humans is just as important as the consideration of actions of humans. ii The thesis of Steven Michael Ammerman is approved. Nancy E. Levine P. Jeffrey Brantingham Monica L. Smith, Committee Chair University of California, Los Angeles 2017 iii Table of Contents 1. -

Paleogenomics of Animal Domestication
Paleogenomics of Animal Domestication Evan K. Irving-Pease, Hannah Ryan, Alexandra Jamieson, Evangelos A. Dimopoulos, Greger Larson, and Laurent A. F. Frantz Abstract Starting with dogs, over 15,000 years ago, the domestication of animals has been central in the development of modern societies. Because of its importance for a range of disciplines – including archaeology, biology and the humanities – domestication has been studied extensively. This chapter reviews how the field of paleogenomics has revolutionised, and will continue to revolutionise, our under- standing of animal domestication. We discuss how the recovery of ancient DNA from archaeological remains is allowing researchers to overcome inherent shortcom- ings arising from the analysis of modern DNA alone. In particular, we show how DNA, extracted from ancient substrates, has proven to be a crucial source of information to reconstruct the geographic and temporal origin of domestic species. We also discuss how ancient DNA is being used by geneticists and archaeologists to directly observe evolutionary changes linked to artificial and natural selection to generate a richer understanding of this fascinating process. Keywords Ancient DNA · Archaeology · Domestication · Entomology · Evolution · Genomics · Zoology E. K. Irving-Pease (*) · H. Ryan · A. Jamieson · E. A. Dimopoulos · G. Larson The Palaeogenomics and Bio-Archaeology Research Network, Research Laboratory for Archaeology and History of Art, University of Oxford, Oxford, UK e-mail: [email protected] L. A. F. Frantz (*) The Palaeogenomics and Bio-Archaeology Research Network, Research Laboratory for Archaeology and History of Art, University of Oxford, Oxford, UK School of Biological and Chemical Sciences, Queen Mary University of London, London, UK e-mail: [email protected] Charlotte Lindqvist and Om P. -

Patterns and Power of Phenotypic Selection in Nature
Articles Patterns and Power of Phenotypic Selection in Nature JOEL G. KINGSOLVER AND DAVID W. PFENNIG Phenotypic selection occurs when individuals with certain characteristics produce more surviving offspring than individuals with other characteristics. Although selection is regarded as the chief engine of evolutionary change, scientists have only recently begun to measure its action in the wild. These studies raise numerous questions: How strong is selection, and do different types of traits experience different patterns of selection? Is selection on traits that affect mating success as strong as selection on traits that affect survival? Does selection tend to favor larger body size, and, if so, what are its consequences? We explore these questions and discuss the pitfalls and future prospects of measuring selection in natural populations. Keywords: adaptive landscape, Cope’s rule, natural selection, rapid evolution, sexual selection henotypic selection occurs when individuals with selection on traits that affect survival stronger than on those Pdifferent characteristics (i.e., different phenotypes) that affect only mating success? In this article, we explore these differ in their survival, fecundity, or mating success. The idea and other questions about the patterns and power of phe- of phenotypic selection traces back to Darwin and Wallace notypic selection in nature. (1858), and selection is widely accepted as the primary cause of adaptive evolution within natural populations.Yet Darwin What is selection, and how does it work? never attempted to measure selection in nature, and in the Selection is the nonrandom differential survival or repro- century following the publication of On the Origin of Species duction of phenotypically different individuals. -

Animal Domestication in the Era of Ancient Genomics Laurent Frantz, Daniel Bradley, Greger Larson, Ludovic Orlando
Animal domestication in the era of ancient genomics Laurent Frantz, Daniel Bradley, Greger Larson, Ludovic Orlando To cite this version: Laurent Frantz, Daniel Bradley, Greger Larson, Ludovic Orlando. Animal domestication in the era of ancient genomics. Nature Reviews Genetics, Nature Publishing Group, 2020, 21 (8), pp.449-460. 10.1038/s41576-020-0225-0. hal-03030302 HAL Id: hal-03030302 https://hal.archives-ouvertes.fr/hal-03030302 Submitted on 30 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Animal domestication in the era of ancient genomics Laurent A. F. Frantz1†, Daniel G. Bradley2, Greger Larson3 and Ludovic Orlando4,5† 1 School of Biological and Chemical Sciences, Queen Mary University of London, London, UK. 2 Smurfit Institute of Genetics, Trinity College Dublin, Dublin, Ireland 3 The Palaeogenomics & Bio-Archaeology Research Network, Research Laboratory for Archaeology and History of Art, The University of Oxford, Oxford, UK. 4 Laboratoire d’Anthropobiologie Moléculaire et d’Imagerie de Synthèse, CNRS UMR 5288, Université de Toulouse, -

Arise by Chance As the Result of Mutation. They Therefore Suggest
THE EVOLUTION OF DOMINANCE UNDER DISRUPTIVE SELECTION C. A. CLARKE and P. Ni. SHEPPARD Department of Medicine and Department of Zoology, University of Liverpool Received6.iii.59 1.INTRODUCTION INa paper on the effects of disruptive selection, Mather (1955) pointed out that if there are two optimum values for a character and all others are less advantageous or disadvantageous there will be disruptive selection which can lead to the evolution of a polymorphism. Sheppard (1958) argued that where such selection is effective and the change from one optimum value to the other is switched by a single pair of allelomorphs there will be three genotypes but only two advantageous phenotypes. Consequently if dominance were absent initially it would be evolved as a result of the disruptive selection, the heterozygote and one of the homozygotes both coming to resemble one of the two optimum phenotypes (see Ford, 1955, on Tripharna comes). Thoday (1959) has shown by means of an artificial selection experiment that, even when a character is, at the beginning, controlled polygenically (sternopleural chaeta-number in Drosophila) and there is 50 per cent. gene exchange between the "high" and "low" selected sub-popu- lations, a polymorphism can evolve. The most fully understood examples of disruptive selection (other than sex) are provided by instances of Batesian Mimicry, where there are a number of distinct warningly coloured species, acting as models, which are mimicked by the polymorphic forms of a single more edible species. Fisher and Ford (see Ford, 1953) have argued that a suffi- ciently good resemblance between mimic and model is not likely to arise by chance as the result of mutation. -
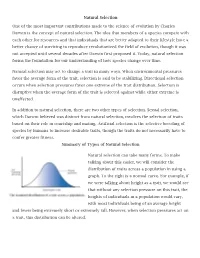
Natural Selection One of the Most Important Contributions Made to The
Natural Selection One of the most important contributions made to the science of evolution by Charles Darwin is the concept of natural selection. The idea that members of a species compete with each other for resources and that individuals that are better adapted to their lifestyle have a better chance of surviving to reproduce revolutionized the field of evolution, though it was not accepted until several decades after Darwin first proposed it. Today, natural selection forms the foundation for our understanding of how species change over time. Natural selection may act to change a trait in many ways. When environmental pressures favor the average form of the trait, selection is said to be stabilizing. Directional selection occurs when selection pressures favor one extreme of the trait distribution. Selection is disruptive when the average form of the trait is selected against while either extreme is unaffected. In addition to natural selection, there are two other types of selection. Sexual selection, which Darwin believed was distinct from natural selection, involves the selection of traits based on their role in courtship and mating. Artificial selection is the selective breeding of species by humans to increase desirable traits, though the traits do not necessarily have to confer greater fitness. Summary of Types of Natural Selection Natural selection can take many forms. To make talking about this easier, we will consider the distribution of traits across a population in using a graph. To the right is a normal curve. For example, if we were talking about height as a trait, we would see that without any selection pressure on this trait, the heights of individuals in a population would vary, with most individuals being of an average height and fewer being extremely short or extremely tall. -

Domestication Syndrome” in Mammals: a Unified Explanation Based on Neural Crest Cell Behavior and Genetics
HIGHLIGHTED ARTICLE PERSPECTIVES The “Domestication Syndrome” in Mammals: A Unified Explanation Based on Neural Crest Cell Behavior and Genetics Adam S. Wilkins,*,†,1 Richard W. Wrangham,*,‡ and W. Tecumseh Fitch§ *Stellenbosch Institute of Advanced Study, Stellenbosch 7600, South Africa, †Institute of Theoretical Biology, Humboldt University zu Berlin, Berlin 10115, Germany, ‡Department of Human Evolutionary Biology, Harvard University, Cambridge, Massachusetts 02138, and §Department of Cognitive Biology, University of Vienna, A-1090 Vienna, Austria ABSTRACT Charles Darwin, while trying to devise a general theory of heredity from the observations of animal and plant breeders, discovered that domesticated mammals possess a distinctive and unusual suite of heritable traits not seen in their wild progenitors. Some of these traits also appear in domesticated birds and fish. The origin of Darwin’s “domestication syndrome” has remained a conundrum for more than 140 years. Most explanations focus on particular traits, while neglecting others, or on the possible selective factors involved in domestication rather than the underlying developmental and genetic causes of these traits. Here, we propose that the domestication syndrome results predominantly from mild neural crest cell deficits during embryonic development. Most of the modified traits, both morphological and physiological, can be readily explained as direct consequences of such deficiencies, while other traits are explicable as indirect consequences. We first show how the hypothesis can account for the multiple, apparently unrelated traits of the syndrome and then explore its genetic dimensions and predictions, reviewing the available genetic evidence. The article concludes with a brief discussion of some genetic and developmental questions raised by the idea, along with specific predictions and experimental tests.