Navassa Island the State of Coral Reef Ecosystems of Navassa Island Margaret W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OGC-98-5 U.S. Insular Areas: Application of the U.S. Constitution
United States General Accounting Office Report to the Chairman, Committee on GAO Resources, House of Representatives November 1997 U.S. INSULAR AREAS Application of the U.S. Constitution GAO/OGC-98-5 United States General Accounting Office GAO Washington, D.C. 20548 Office of the General Counsel B-271897 November 7, 1997 The Honorable Don Young Chairman Committee on Resources House of Representatives Dear Mr. Chairman: More than 4 million U.S. citizens and nationals live in insular areas1 under the jurisdiction of the United States. The Territorial Clause of the Constitution authorizes the Congress to “make all needful Rules and Regulations respecting the Territory or other Property” of the United States.2 Relying on the Territorial Clause, the Congress has enacted legislation making some provisions of the Constitution explicitly applicable in the insular areas. In addition to this congressional action, courts from time to time have ruled on the application of constitutional provisions to one or more of the insular areas. You asked us to update our 1991 report to you on the applicability of provisions of the Constitution to five insular areas: Puerto Rico, the Virgin Islands, the Commonwealth of the Northern Mariana Islands (the CNMI), American Samoa, and Guam. You asked specifically about significant judicial and legislative developments concerning the political or tax status of these areas, as well as court decisions since our earlier report involving the applicability of constitutional provisions to these areas. We have included this information in appendix I. 1As we did in our 1991 report on this issue, Applicability of Relevant Provisions of the U.S. -
Agroforestry: Enhancing Resiliency in U.S
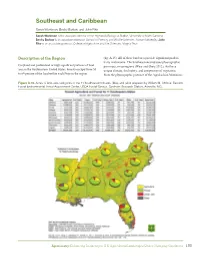
Southeast and Caribbean Sarah Workman, Becky Barlow, and John Fike Sarah Workman is the associate director of the Highlands Biological Station, University of North Carolina; Becky Barlow is an associate professor, School of Forestry and Wildlife Sciences, Auburn University; John Fike is an associate professor, College of Agriculture and Life Sciences, Virginia Tech. Description of the Region (fig. A.15). All of these land uses provide significant produc- tivity and income. The Southeast encompasses physiographic Cropland and pastureland occupy significant portions of land provinces, or ecoregions (Wear and Greis 2012), that have area in the Southeastern United States. Forests occupy from 50 unique climate, fire history, and composition of vegetation. to 69 percent of the land within each State in the region From the physiographic province of the Appalachian Mountains Figure A.15. Acres of landuse categories of the 11 Southeastern States. (Map and table prepared by William M. Christie, Eastern Forest Environmental Threat Assessment Center, USDA Forest Service, Southern Research Station, Asheville, NC). Agroforestry: Enhancing Resiliency in U.S. Agricultural Landscapes Under Changing Conditions 189 to the alluvial plains of the Mis sissippi River Basin, within land use outside developed zones is perhaps best viewed in deciduous forests of Kentucky and Tennessee and the Interior terms of the nature of woody plant cover and whether animals Highlands of the Ozarks, to the Piedmont, Flatwoods, and are excluded or allowed access. Both Puerto Rico and the U.S. Coastal Plains, a large portion of the land area is appropriate Virgin Islands are experiencing a trend toward an increase in for implementing several types of agroforestry, integrating woody cover with the loss of agricultural land and pastureland either crops or livestock, or both, with trees and woody (Brandeis and Turner 2013a, 2013b; Brandeis et al. -

Download Table 93
National Center for Science and Engineering Statistics | NSF 21-329 TABLE 93 Federal obligations for R&D plant, by state or location and selected agency: FY 2019 (Dollars in thousands) State or location Total DHS DOC DOD DOE DOI DOT EPA HHS NASA NSF USDA All locations 4,329,453.9 1,306.0 464,332.3 609,655.9 2,245,499.5 4,646.5 39,261.0 4,695.0 243,000.0 35,925.4 652,140.0 28,992.4 Alabama 125,340.2 0.0 0.0 122,721.8 0.0 0.0 529.4 0.0 0.0 0.0 2,089.0 0.0 Alaska 6,648.3 0.0 6,287.0 54.9 0.0 0.0 0.0 0.0 0.0 0.0 306.4 0.0 Arizona 12,537.1 0.0 0.0 262.3 0.0 0.0 8.8 0.0 5,045.7 0.0 6,445.1 775.2 Arkansas 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 California 676,511.6 0.0 4,062.0 67,315.7 544,473.1 0.0 1,023.7 0.0 0.0 15,377.1 38,278.0 5,982.0 Colorado 365,052.1 0.0 17,195.1 34,880.0 305,460.0 0.0 1,679.6 0.0 0.0 0.0 4,115.1 1,722.2 Connecticut 1,481.4 1,306.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 175.4 0.0 Delaware 9,559.9 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 9,559.9 0.0 District of Columbia 150,500.7 0.0 0.0 37,780.9 23,000.0 0.0 4,937.4 913.6 0.0 0.0 83,755.3 113.5 Florida 33,775.9 0.0 14,211.0 4,742.5 0.0 0.0 5.0 165.6 0.0 8,026.6 5,254.8 1,370.5 Georgia 6,592.6 0.0 0.0 0.0 0.0 0.0 0.0 112.3 0.0 0.0 2,681.6 3,798.6 Hawaii 24,289.5 0.0 4,276.0 7,437.1 0.0 0.0 0.0 0.0 6,478.2 0.0 6,098.2 0.0 Idaho 3,395.0 0.0 0.0 2,590.0 400.0 0.0 0.0 0.0 0.0 0.0 405.0 0.0 Illinois 471,335.8 0.0 0.0 16,141.5 444,871.6 0.0 104.3 0.0 3,269.5 0.0 6,949.0 0.0 Indiana 8,852.3 0.0 0.0 2,550.0 0.0 0.0 0.0 0.0 0.0 0.0 6,302.3 0.0 Iowa 2,081.8 0.0 0.0 0.0 1,000.0 0.0 -

ISO Country Codes
COUNTRY SHORT NAME DESCRIPTION CODE AD Andorra Principality of Andorra AE United Arab Emirates United Arab Emirates AF Afghanistan The Transitional Islamic State of Afghanistan AG Antigua and Barbuda Antigua and Barbuda (includes Redonda Island) AI Anguilla Anguilla AL Albania Republic of Albania AM Armenia Republic of Armenia Netherlands Antilles (includes Bonaire, Curacao, AN Netherlands Antilles Saba, St. Eustatius, and Southern St. Martin) AO Angola Republic of Angola (includes Cabinda) AQ Antarctica Territory south of 60 degrees south latitude AR Argentina Argentine Republic America Samoa (principal island Tutuila and AS American Samoa includes Swain's Island) AT Austria Republic of Austria Australia (includes Lord Howe Island, Macquarie Islands, Ashmore Islands and Cartier Island, and Coral Sea Islands are Australian external AU Australia territories) AW Aruba Aruba AX Aland Islands Aland Islands AZ Azerbaijan Republic of Azerbaijan BA Bosnia and Herzegovina Bosnia and Herzegovina BB Barbados Barbados BD Bangladesh People's Republic of Bangladesh BE Belgium Kingdom of Belgium BF Burkina Faso Burkina Faso BG Bulgaria Republic of Bulgaria BH Bahrain Kingdom of Bahrain BI Burundi Republic of Burundi BJ Benin Republic of Benin BL Saint Barthelemy Saint Barthelemy BM Bermuda Bermuda BN Brunei Darussalam Brunei Darussalam BO Bolivia Republic of Bolivia Federative Republic of Brazil (includes Fernando de Noronha Island, Martim Vaz Islands, and BR Brazil Trindade Island) BS Bahamas Commonwealth of the Bahamas BT Bhutan Kingdom of Bhutan -

Guam Demographic Profile Summary File: Technical Documentation U.S
Guam Demographic Profile Summary File Issued March 2014 2010 Census of Population and Housing DPSFGU/10-3 (RV) Technical Documentation U.S. Department of Commerce Economics and Statistics Administration U.S. CENSUS BUREAU For additional information concerning the files, contact the Customer Liaison and Marketing Services Office, Customer Services Center, U.S. Census Bureau, Washington, DC 20233, or phone 301-763-INFO (4636). For additional information concerning the technical documentation, contact the Administrative and Customer Services Division, Electronic Products Development Branch, U.S. Census Bureau, Wash- ington, DC 20233, or phone 301-763-8004. Guam Demographic Profile Summary File Issued March 2014 2010 Census of Population and Housing DPSFGU/10-3 (RV) Technical Documentation U.S. Department of Commerce Penny Pritzker, Secretary Vacant, Deputy Secretary Economics and Statistics Administration Mark Doms, Under Secretary for Economic Affairs U.S. CENSUS BUREAU John H. Thompson, Director SUGGESTED CITATION 2010 Census of Population and Housing, Guam Demographic Profile Summary File: Technical Documentation U.S. Census Bureau, 2014 (RV). ECONOMICS AND STATISTICS ADMINISTRATION Economics and Statistics Administration Mark Doms, Under Secretary for Economic Affairs U.S. CENSUS BUREAU John H. Thompson, Director Nancy A. Potok, Deputy Director and Chief Operating Officer Frank A. Vitrano, Acting Associate Director for Decennial Census Enrique J. Lamas, Associate Director for Demographic Programs William W. Hatcher, Jr., Associate Director for Field Operations CONTENTS CHAPTERS 1. Abstract ............................................... 1-1 2. How to Use This Product ................................... 2-1 3. Subject Locator .......................................... 3-1 4. Summary Level Sequence Chart .............................. 4-1 5. List of Tables (Matrices) .................................... 5-1 6. Data Dictionary .......................................... 6-1 7. -

Navassa National Wildlife Refuge
U.S. Fish & Wildlife Service Navassa National Wildlife Refuge Photos (top to bottom): Refuge Objectives Navassa National Wildlife Refuge. ■ To preserve and protect coral reef ecosystems and the marine Navassa Lighthouse. environment. Red-footed Booby. ■ To restore and enhance native Endemic Navassa Anole. wildlife and plants. Refuge Facts ■ To provide opportunities for Discovered by Columbus in 1498 scientific research. and then rediscovered in the 1856 Management Tools photo: USFWS photo: Guano Rush. The guano deposits were ■ Wildlife monitoring. worked until the company failed in 1898. Navassa became an increased ■ Scientific research. hazard to shipping with the building of the Panama Canal. Consequently, ■ Coral reef monitoring. the U.S. Coast Guard built a 162 foot ■ Vegetation monitoring. lighthouse in 1917. In December 1999, jurisdiction was transferred to Questions and Answers the U.S. Fish and Wildlife Service Is the refuge open to public? (Service) to be managed as a National No. The refuge is closed to the public. Wildlife Refuge (NWR) for the Access is extremely hazardous. There purpose of protecting the unique are no beaches on Navassa. The island ecosystem of Navassa Island, the rises abruptly from the sea with cliffs photo: C. Lombard photo: adjacent coral reefs and marine reaching heights of 20 meters or waters. more. ■ Acres: The Island covers 1,344. What type of research has been The refuge includes a 12 nautical conducted at Navassa? mile radius of marine habitat. Since the refuge’s establishment, investigations and inventories have ■ Location: Navassa Island is located been conducted by personnel from 35 miles west of the Tiburon the Service, NOAA, Smithsonian, Peninsula of Haiti. -

A Report on the State of the Islands 1999
U.S. Department of the Interior OfficeofInsularAffairs AReportonthe StateoftheIslands 1999 Front cover photo compliments of Marshall Islands Visitors Authority Benjamin Graham General Manager State Of The Islands TableOfContents A Report on the State of the Islands ............................................................................. 1 Introduction .......................................................................................................................................... 1 Acknowledgements ................................................................................................................................1 Chapter 1. The Department of the Interior’s Role in the Insular Areas 1.1 The Department’s Evolving Role in Insular Affairs. ................................................................................. 2 1.2 Fiscal Years 1998 and 1999 ................................................................................................................ 2 Office of Insular Affairs .......................................................................................................................... 3 General Technical Assistance ................................................................................................................... 3 Operations and Maintenance Improvement Program ...................................................................................... 3 Insular Management Control Initiative ...................................................................................................... -

46 CFR Ch. II (10–1–18 Edition)
§ 308.502 46 CFR Ch. II (10–1–18 Edition) (d) Imported to, or exported from, the an underwriting agent upon applica- United States, its Territories or posses- tion. sions, under contracts of sale or pur- chase by the terms of which the risk of § 308.504 Definition of territories and loss by war risks or the obligation to possessions. provide insurance against such risks is Whenever reference is made to the assumed by or falls upon a citizen or territories and possessions of the resident of the United States, its Terri- United States in this subpart or in any tories or possessions; supplement thereto or any policy of in- (e) Sold or purchased by citizens or surance issued pursuant to the provi- residents of the United States, its Ter- sions thereof, said territories and pos- ritories or possessions, under contracts sessions shall be deemed to include of sale or purchase by the terms of only the Virgin Islands of the United which the risk of loss by war risks or States, the Commonwealth of Puerto the obligation to provide insurance Rico, American Samoa, Common- against such risks is assumed by or wealth of the Northern Mariana Is- falls upon a citizen or resident of the lands, Guam, Wake Island, Midway Is- United States, its Territories or posses- lands, Baker Island, Howland Island, sions; Jarvis Island, Johnston Atoll, Kingman (f) Shipped between ports in the Reef, Navassa Island, and Wake Island. United States, or between ports in the OPEN POLICY WAR RISK CARGO United States and its Territories and INSURANCE possessions, or between ports in such Territories or possessions; and § 308.505 General. -

Puerto Rico and US Unincorporated Territories
© 2009 BirdLife International Juan de Dios Martínez Mera N35-76 y Av. Portugal Casilla 17-17-717 Quito, Ecuador. Tel: +593 2 2277059 Fax: +593 2 2469838 [email protected] www.birdlife.org BirdLife International is a UK-registered charity No. 1042125 ISBN: 978-9942-9959-0-2 Recommended citation: DEVENISH, C., DÍAZ FERNÁNDEZ, D. F., CLAY, R. P., DAVIDSON, I. & YÉPEZ ZABALA,I.EDS. (2009) Important Bird Areas Americas - Priority sites for biodiversity conservation. Quito, Ecuador: BirdLife International (BirdLife Conservation Series No. 16). To cite this chapter: ANADÓN-IRIZARRY, V., CORVEN, J., MÉNDEZ-GALLARDO, V., SALGUERO-FARÍA, J. A. & SCHWAGERL, J. (2009) Puerto Rico and US Unincorporated Territories. Pp 317 – 328 in C. Devenish, D. F. Díaz Fernández, R. P. Clay, I. Davidson & I. Yépez Zabala Eds. Important Bird Areas Americas - Priority sites for biodiversity conservation. Quito, Ecuador: BirdLife International (BirdLife Conservation Series No. 16). The purpose of the information contained in this book is to support conservation initiatives in the Americas, for which it may be reproduced. Using this information for commercial purposes is not permitted. If part or all of this information is used or included in any other publication, BirdLife International must be cited as copyright holder. Those who provided illustrations or photographs in this book have copyright over them and these are not permitted to be reproduced separately to the texts accompanying them. The presentation of material in this book and the geographical designations employed do not imply the expression of any opinion whatsoever on the part of BirdLife International concerning the legal status of any country, territory or area, or concerning the delimitation of its frontiers or boundaries. -

National Wildlife Refuge System
U.S. Fish & Wildlife Service National Wildlife Refuge System o o o o o o o o o o o o o o o o o o o o o 145 150 155 160 165 170 175 E 180 o 70 o 175 W 170 165 160 155 150 145 140 135 130 70 125 120 115 Barrow A E A R C T I S Midway Atoll NWR C C O C E A N H R T 30 o U F O E A U Papahānaumokuākea MNM K e B C m i� ar Teshekpuk PACIFIC ISLANDS H Lake Prudhoe Bay M a H I k a s 0 w a 200 400 MILES ai l ian A Islan S ds NW E Marianas Trench MNM (Island Unit) 0 300 600 KILOMETERS R ) t A i n U R TROPIC OF CANCER o SCALE 1:29,000,000 c i R n o o 65 a Albers equal area projection, standard parallels 7 S and 20 N, U c Colville l o o Alaska Mari�me V central meridian 174 30' E S ( Honolulu Arc�c M H S N A 55 W M A o o I 20 h I I c Noatak n A e r T See inset map of s a Hawaii below R n a i A r RC a TIC C M IR C / LE R K R Guam NWR o t Kobuk e W z NORTHERN MARIANA e A in N b p Wake Atoll NWR and l u e u a c r ISLANDS r i e s Po F Pacific Remote Islands MNM k (UNITED STATES) a f t S R o i o M u c a r r n a Selawik A d r t Yukon Flats i Yukon a S � n m a i r Johnston Atoll NWR and Alaska Mari�me e a N Kanu� M Pacific Remote Islands MNM Mariana Trench NWR / Marianas Trench MNM (Trench Unit) A n g E r i R C B e O 10 o N M R A oyukuk R I C K S H A I F Koyukuk L L C A I S P L Fairbanks A Nome N Kingman Reef NWR and FED D ERA S A Nowitna TED Pacific Remote Islands MNM l Tanana STA Palmyra Atoll NWR and a TES sk A OF a MI Pacific Remote Islands MNM Innoko D CRO M NE St. -

AAPI National Historic Landmarks Theme Study Essay 12
National Park Service U.S. Department of the Interior A National Historic Landmarks Theme Study ASIAN AMERICAN PACIFIC ISLANDER ISLANDER AMERICAN PACIFIC ASIAN Finding a Path Forward ASIAN AMERICAN PACIFIC ISLANDER NATIONAL HISTORIC LANDMARKS THEME STUDY LANDMARKS HISTORIC NATIONAL NATIONAL HISTORIC LANDMARKS THEME STUDY Edited by Franklin Odo Use of ISBN This is the official U.S. Government edition of this publication and is herein identified to certify its authenticity. Use of 978-0-692-92584-3 is for the U.S. Government Publishing Office editions only. The Superintendent of Documents of the U.S. Government Publishing Office requests that any reprinted edition clearly be labeled a copy of the authentic work with a new ISBN. Library of Congress Cataloging-in-Publication Data Names: Odo, Franklin, editor. | National Historic Landmarks Program (U.S.), issuing body. | United States. National Park Service. Title: Finding a Path Forward, Asian American and Pacific Islander National Historic Landmarks theme study / edited by Franklin Odo. Other titles: Asian American and Pacific Islander National Historic Landmarks theme study | National historic landmark theme study. Description: Washington, D.C. : National Historic Landmarks Program, National Park Service, U.S. Department of the Interior, 2017. | Series: A National Historic Landmarks theme study | Includes bibliographical references and index. Identifiers: LCCN 2017045212| ISBN 9780692925843 | ISBN 0692925848 Subjects: LCSH: National Historic Landmarks Program (U.S.) | Asian Americans--History. | Pacific Islander Americans--History. | United States--History. Classification: LCC E184.A75 F46 2017 | DDC 973/.0495--dc23 | SUDOC I 29.117:AS 4 LC record available at https://lccn.loc.gov/2017045212 For sale by the Superintendent of Documents, U.S. -

Travel Insurance Frequently Asked Questions
Risk Management 12601 Aquarius Agora Dr. Orlando, FL 32816-3400 Travel Insurance Frequently Asked Questions STUDENTS Am I covered under this insurance? What do I need to do to get coverage? Yes, students are covered under this policy if you are on a university sponsored trip outside of the United States. This can include a study abroad program, a trip where you are receiving credit hours towards your degree, or a trip with a faculty member or staff on university business. For coverage to be effective, you MUST register through the Travel Registry. To do so, please visit the UCF Global, Study Abroad Office. While on my Study Abroad program, I plan on going snorkeling, one weekend with a few locals, am I covered? Yes, under this policy you are covered, up to 10 days, for side trip(s) that you take which are not part of the university sponsored trip. On your website it says that this insurance is for those traveling outside of the United States. If I am traveling to Puerto Rico, would that be considered traveling outside of the United States? No, the policy excludes coverage for travel within the United States, its territories and possessions, which includes the continental United States, Alaska, Hawaii, Puerto Rico, Federated States of Micronesia, Guam, Marshall Islands, Northern Mariana Islands, Palau, American Samoa, U.S. Virgin Islands, Midway Island, Wake Island, Palmyra Island, Howland Island, Johnston Island, Baker Island, Kingman Reef, Jarvis Island, Navassa Island, and other U.S. islands, cays, and reefs that are not part of any of the fifty states.