International Partners of ICMR an Overview of International
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Workshop Is Designed for Following Completed Registration Form Has to Be Forwarded GGCC LLPP Workshop Professionals: to Workshop Co-Ordinator Along with Prescribed
WHO SHOULD PARTICIPATE? REGISTRATION This workshop is designed for following Completed Registration form has to be forwarded GGCC LLPP Workshop professionals: to Workshop Co-ordinator along with prescribed 18-20 June 2010 Microbiologists, Pathologists and Biochemists. Fee of Rs.3000 in the form of DD. DD should be Lab Directors / Managers and Laboratory drawn in favor of “YRG CARE” payable at Chennai. Technologists. The fee includes registration, workshop materials, QA/QC personnel, Quality Officers and Quality refreshments, breakfast and lunch provided during OVERVIEW Managers. the workshop. GCLP outline the principles and procedures to be Professionals associated with clinical The seats are restricted to 60 and participants will followed by medical laboratories involved in patient laboratory management and accreditation. be registered on “first come-first served basis” . care and/or clinical research so as to provide Only those who are currently engaged in the The registration DOES NOT cover accommodation, consistent, reproducible, auditable, and reliable diagnostic lab or clinical research are encouraged however, assistance will be provided for laboratory results; which contribute to good patient to participate in this workshop and students are arrangement of accommodation on request. not eligible. care and promote a positive attitude toward testing from a patient’s perspective. This workshop is WORKSHOP CONTENTS VENUE designed to offer comprehensive guidance for The key contents of the workshop are: those who are implementing GCLP in their TICEL Bio Park Ltd Principles of quality essentials Taramani Road, Taramani laboratories. QA/QC practices Chennai - 600113 Establishment and management of quality LEARNING OBJECTIVES ORGANIZING COMMITTEE system Documentation structure and system Organizing Chair Learn GCLP principles and their relation to Test facility operation Prof. -

Laboratories Reporting to ICMR
भारतीय आयु셍वज्ञि ान अनुसंधान पररषद वा्य अनुसंधान 셍वभाग, वा्य और पररवार क쥍याण मंत्रालय, भारत सरकार Indian Council of Medical Research Department of Health Research, Ministry of Health and Family Welfare, Government of India Date: 28/07/2021 Total Operational (initiated independent testing) Laboratories reporting to ICMR: Government laboratories : 1297 Private laboratories : 1494 - Real-Time RT PCR for COVID-19 : 1705 (Govt: 623 + Private: 1082) - TrueNat Test for COVID-19 : 938 (Govt: 625 + Private: 313) - CBNAAT Test for COVID-19 : 130 (Govt: 41 + Private: 89) - Other Molecular-Nucleic Acid (M-NA) Testing Platforms for COVID-19 : 18 (Govt: 08 + Private: 10) Note: Other Molecular-Nucleic Acid includes Abbott ID NOW, RT-LAMP, CRISPR-Cas9 and Accula™ Total No. of Labs : 2791 *CSIR/DBT/DST/DAE/ICAR/DRDO/MHRD/ISRO Laboratories. #Laboratories approved for both Real-Time RT-PCR and TrueNat/CBNAAT $Laboratories approved for both TrueNAT and CBNAAT ¥ Laboratories approved for Abbott ID NOW alone or in combination with any other testing platforms @Laboratories approved for RT-LAMP alone or in combination with any other testing platforms € Laboratories approved for CRISPR-Cas9 alone or in combination with any other testing platforms δ Laboratories approved for Accula™ alone or in combination with any other testing platforms P: Provisional Δ Pvt. Laboratories acquired by Govt. 1 | P a g e S. Test Names of States Names of Government Institutes Names of Private Institutes No. Category 1. Andhra Pradesh RT-PCR 1. Sri Venkateswara Institute of Medical 1. Manipal Hospital, Tadepalli, Guntur (131) Sciences, Tirupati 2. -

List of Abbreviations
LIST OF ABBREVIATIONS S. No. 1. A&N Andaman & Nicobar 2. ACO Assistant Committee Officer 3. AEES Atomic Energy Education Society 4. AeBAS Aadhaar enabled Biometric Attendance System 5. AIIMS All India Institute of Medical Sciences 6. AIU Association of Indian Universities 7. AMC Annual Maintenance Contract 8. ARO Assistant Research Officer 9. ASEAN Association of South-East Asian Nations 10. ASGP Association of Secretaries-General of Parliaments 11. ASI Archaeological Survey of India 12. ASSOCHAM Associated Chambers of Commerce and Industry of India 13. ATNs Action Taken Notes 14. ATRs Action Taken Reports 15. AWS Automatic Weather Station 16. AYCL Andrew Yule & Company Ltd. 17. AYUSH Ayurvedic, Yoga and Naturopathy, Unani, Siddha and Homeopathy 18. BCD Basic Customs Duty 19. BEML Bharat Earth Movers Limited 20. BHAVINI Bhartiya Nabhikiya Vidyut Nigam Ltd. 21. BHEL Bharat Heavy Electricals Ltd. 22. BHMRC Bhopal Memorial Hospital & Research Centre 23. BIOS Bills Information Online System 24. BIS Bureau of Indian Standards 25. BMRCL Bangalore Metro Rail Corporation Ltd. 26. BOAT Board of Apprentice Ship Training 27. BOB Bank of Baroda 28. BPCL Bharat Petroleum Corporation Limited 29. BPST Bureau of Parliamentary Studies and Training 30. BRO Border Roads Organisation 31. BSF Border Security Force 32. BSNL Bharat Sanchar Nigam Limited 33. C&AG Comptroller & Auditor General 34. CARA Central Adoption Resource Authority 35. CAT Central Administrative Tribunal 36. CBI Central Bureau of Investigation 37. CBRN Chemical Biological Radiological Nuclear 38. CBDT Central Board of Direct Taxes 39. CCL Child Care Leave 40. CCRYN Central Council for Research in Yoga and Naturopathy 41. CCS Central Civil Services 42. -

EPORT 2017 -18 of TATA MEMORIAL CENTRE (A Grant-In-Aid Institute of the Department of Atomic Energy, Government of India)
ANNUAL REPORT 2017 -18 of TATA MEMORIAL CENTRE (A Grant-in-Aid Institute of the Department of Atomic Energy, Government of India) Tata Memorial Hospital, Mumbai. Advanced Centre for Treatment, Research and Education in Cancer, Navi Mumbai. Centre for Cancer Epidemiology, Navi Mumbai. Homi Bhabha Cancer Hospital and Research Centre, Visakhapatnam. Homi Bhabha Cancer Hospital, Sangrur. Homi Bhabha Cancer Hospital and Research Centre, Mohali. Dr. Bhubaneswar Borooah Cancer Institute, Guwahati. Homi Bhabha Cancer Hospital, Varanasi. Mahamana Pandit Madan Mohan Malviya Cancer Centre, Varanasi. Tata Memorial Centre Mission and Vision of the Tata Memorial Centre Mission The Tata Memorial Centre’s mission is to provide comprehensive cancer care to one and all, through its motto of excellence in service, education and research. Vision As the premier cancer centre in the country, we will provide leadership in guiding the national policy and strategy for cancer care by: Promoting outstanding services through evidence based practice of oncology Commitment of imparting education in cancer to students, trainees, professionals, employees and the public and, Emphasis on research that is affordable, innovative and relevant to the needs of the country. Tata Memorial Centre, Annual Report 2017 - 2018 Contents Tata Memorial Centre (TMC) Governing Council ...................................................................................... 9 Messages Director TMC .............................................................................................. -

Institutions Funded by Dae
GOVERNMENT OF INDIA DEPARTMENT OF ATOMIC ENERGY RAJYA SABHA STARRED QUESTION NO. 161 TO BE ANSWERED ON 11.08.2011 INSTITUTIONS FUNDED BY DAE 161 . SHRIMATI RENUBALA PRADHAN: Will the PRIME MINISTER be pleased to state: (a) the details of the institutions funded by the Department of Atomic Energy and the amount of plan and non-plan funds allocated to them so far during the last three years; (b) whether any achievements have been made by each such institution during the last three years; (c) if so, whether such achievements are of international repute; and (d) the details thereof, institution-wise, during the last three years? ANSWER THE MINISTER OF STATE FOR PERSONNEL, PUBLIC GRIEVANCES & PENSIONS AND IN THE PRIME MINISTER’S OFFICE (SHRI V. NARAYANASAMY) (a) to (d) A statement is laid on the Table of the House. ******* STATEMENT REFERRED TO IN REPLY TO RAJYASABHA STARRED QUESTION NO.161 FOR ANSWER ON 11.08.2011 BY SMT. RENUBALA PRADHAN REGARDING INSTITUTIONS FUNDED BY DAE (a) The details are given in Annexure 1; (b) Yes, Sir; (c) Yes, Sir; (d) The details are given in Annexure 2. ******* Annexure-1 The Aided Institutions under DAE are: 1. Tata Institute of Fundamental Research (TIFR), Mumbai 2. Tata Memorial Centre (TMC), Mumbai 3. Saha Institute of Nuclear Physics (SINP), Kolkata 4. Institute of Physics (IoP), Bhubaneswar 5. Institute of Mathematical Sciences (IMSc), Chennai 6. Harish Chandra Research Institute (HRI), Allahabad 7. Institute for Plasma Research (IPR), Gandhinagar 8. National Institute of Science, Education and Research (NISER), Bhubaneswar 9. Atomic Energy Education Society (AEES), Mumbai Details of Grants given to Aided Institutions under DAE for the period 2008-09 to 2011-12 under Plan and Non-Plan (` in crores) Aided Institutions Sr. -

Communication Dated 26 September 2008, Copied to the Agency by the Permanent Mission of India Regarding the Middle
s^llAEA Atoms for Peace Information Circular INFCIRC/731 Date: 25 July 2008 General Distribution Original: English Communication dated 25 July 2008 received from the Permanent Mission of India concerning a document entitled "Implementation of the India-United States Joint Statement of July 18, 2005: India's Separation Plan" The Secretariat has received a communication dated 25 July 2008 from the Permanent Mission of India to the Agency, attaching a document entitled "Implementation of the India-United States Joint Statement of July 18, 2005: India's Separation Plan". As requested by the Permanent Mission of India to the Agency, the communication and its attachment are herewith circulated for information. INFCIRC/731 Attachment $ m-*a *T jarift Tun* Permanent Mission of India to !'>•' International Organisations in Vienna Karnlnoirmg ? ™«**« A.,»W VIENNA No. Vicn/J IO/I7/07 25,h July 2008 I he Permanent Mission of India in Vienna presents its compliments to UlC Director-General of the Inlernalional Atomic Energy Agency (IAFA) and has the honour to enclose a document entitled "Implementation of the India-United Stales Joint Statement of July 18, 2005: India's Separation Plan.". It is the Government of India's intention to move forward in accordance with the provisions of the "Agreement between the Government of India and the International Atomic Energy Agency for the Application of Safeguards to Civilian Nuclear Facilities" reproduced as an attachment to the agenda item GOV/2008'30 dated 9 July 2008. aftci its entry into force. The Permanent Mission of India in Vienna requests the Agency lo distribute this letter along with the enclosed document to all Memhcr-Sialcs of the Agency for information. -

Cme Invitation Design
AUTOIMMUNE DISEASES Diagnosis and Interpretation Organized By Date March 23, 2014 Venue Microbiological Laboratory IMA Hall, (NABL Accredited), Coimbatore Syrian Church Road, & Coimbatore Indian Association of Medical Microbiologists Time Tamil Nadu Pondicherry Chapter 9am - 6pm Mariappa Mani Chairman-CME, Managing Director-Microbiological Laboratory .................................................................................................................................................. In an autoimmune reaction, the immune system attacks thyself by mistake and harms the body's own tissues. The autoimmune diseases comprise a group of immunologic disorders whose common denominator is the presence of an idiopathic systemic autoimmune process. The MICROBIOLOGICAL LABORATORY, Coimbatore provides a comprehensive panel of diagnostic services using the latest scientific discoveries to provide innovative methods in the detection, diagnosis and treatment assessment of immunologic, hematologic, pathologic, biochemical and oncologic disease states. A quiet revolution in the field of autoimmunity is underway. Its objective is to provide simple and accurate results for making laboratory diagnosis of autoimmune diseases. Although the autoimmune diseases share many common features and clinical presentations, differentiation between the diseases is crucial because of important distinction in clinical course, appropriate treatment and prognosis. Hence, there is urgent need to review and update the current scenario on clinical manifestation, laboratory -

Stamps of India - Commemorative by Prem Pues Kumar [email protected] 9029057890
E-Book - 26. Checklist - Stamps of India - Commemorative By Prem Pues Kumar [email protected] 9029057890 For HOBBY PROMOTION E-BOOKS SERIES - 26. FREE DISTRIBUTION ONLY DO NOT ALTER ANY DATA ISBN - 1st Edition Year - 1st May 2020 [email protected] Prem Pues Kumar 9029057890 Page 1 of 76 Nos. YEAR PRICE NAME Mint FDC B. 1 2 3 1947 1 21-Nov-47 31/2a National Flag 2 15-Dec-47 11/2a Ashoka Lion Capital 3 15-Dec-47 12a Aircraft 1948 4 29-May-48 12a Air India International 5 15-Aug-48 11/2a Mahatma Gandhi 6 15-Aug-48 31/2a Mahatma Gandhi 7 15-Aug-48 12a Mahatma Gandhi 8 15-Aug-48 10r Mahatma Gandhi 1949 9 10-Oct-49 9 Pies 75th Anni. of Universal Postal Union 10 10-Oct-49 2a -do- 11 10-Oct-49 31/2a -do- 12 10-Oct-49 12a -do- 1950 13 26-Jan-50 2a Inauguration of Republic of India- Rejoicing crowds 14 26-Jan-50 31/2a Quill, Ink-well & Verse 15 26-Jan-50 4a Corn and plough 16 26-Jan-50 12a Charkha and cloth 1951 17 13-Jan-51 2a Geological Survey of India 18 04-Mar-51 2a First Asian Games 19 04-Mar-51 12a -do- 1952 20 01-Oct-52 9 Pies Saints and poets - Kabir 21 01-Oct-52 1a Saints and poets - Tulsidas 22 01-Oct-52 2a Saints and poets - MiraBai 23 01-Oct-52 4a Saints and poets - Surdas 24 01-Oct-52 41/2a Saints and poets - Mirza Galib 25 01-Oct-52 12a Saints and poets - Rabindranath Tagore 1953 26 16-Apr-53 2a Railway Centenary 27 02-Oct-53 2a Conquest of Everest 28 02-Oct-53 14a -do- 29 01-Nov-53 2a Telegraph Centenary 30 01-Nov-53 12a -do- 1954 31 01-Oct-54 1a Stamp Centenary - Runner, Camel and Bullock Cart 32 01-Oct-54 2a Stamp Centenary -
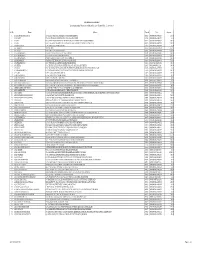
Format Section
ION EXCHANGE LIMITED List Of Outstanding Warrant as on 15th October, 2016 (Payment Date:- IO 14-09-2016) Sr. No. Name Address Pincode Folio Amount 1 A ANANDA RANJANA DOS 32, THOMAS NAGAR LITTLE MOUNT SAIDAPET MADRAS 600015 0000000000IOA0002323 162.00 2 A G KAISER C/O MOHD ISMAIL HUNDEKAR STATION ROAD YADGIR 585202 0000000000IOA0001776 84.00 3 A GOPAL H 14/3 HOUSING BOARD FLAT SOUTH SIVAN KOIL STREET VADAPALANI CHENNAI 600026 0000000000IOA0002592 300.00 4 A GOPAL H 14/3, HOUSING BOARD FLAT SOUTH SIVAN KOIL STREET VADAPALANI CHENNAI 600026 0000000000IOA0002553 150.00 5 A H SRINIVASAN 1/34 ASHOK NAGAR NEW DELHI 110018 0000000000IOA0001370 3.00 6 A K BHARAT TEST DUMMY 999999 0000000000IOA0011001 375.00 7 A K KHOLI K-16 LAJPAT NAGAR NEW DELHI 110024 0000000000IOA0011160 1,659.00 8 A L SUBRAMANIAN POCKET B-122 MAYUR VIHAR PHASE II DELHI 110091 0000000000IOA0002143 300.00 9 A L SUBRAMANIAN LT 15A DDA FLATS KALKAJI, NEW DELHI 110019 0000000000IOA0010003 282.00 10 A L SUBRAMANIAN B-122 POCKET B MAYUR VIHAR PHASE II N DELHI 110091 0000000000IOA0011124 300.00 11 A MANONMANI 6 RAILWAY FEEDER ROAD SULUR (P O) COIMBATORE 641402 0000000000IOA0000669 546.00 12 A PADMANABHAN 24/16 VEDACHALA GARDEN MANDAVELI CHENNAI 600028 0000IN30108022159972 600.00 13 A R SEETHA 15-1C RENGANATHAPURAM OFFICERS COLONY, ATSHAYA APP TRICHY 620017 00001203840000221028 300.00 14 A RAMAN FLAT NO 5, GROUND FLOOR NAVIN APARTMENT HANUMAN NAGAR, KATEMANDI KALYAN 421306 0000000000IOA0010015 84.00 15 A S CHANDRASHEKAR ION EXCHANGE INDIA LTD 2ND FLR,NEETA TOWERS OPP SANDVIK ,DAPODI -

List of Stamps from 1852 Onwards
LIST OF STAMPS FROM 1852 ONWARDS POSTAGE STAMPS – PRE-INDEPENDENCE Year Denomination Particulars 1 1852 /2a SCINDE DAWK 1 1854 /2a EAST INDIA CO, ISSUES 1a -do- 4a -do- 1854 4a QUEEN VICTORIA 1 /2a -do- 1a -do- 2a -do- 1855 4a -do- 8a -do- 1 1856-64 /2a -do- 1a -do- 2a -do- 4a -do- 8a -do- UNDER THE CROWN - QUEEN 1860 8p VICTORIA 1 1865 /2a Elephant’s Head Watermark 8p -do- 1a -do- 2a -do- 4a -do- 8a -do- 1866 6a -do- 1866-67 4a Octagonal design 6a8p -do- 1868 8a Die II 1 1873 /2a -do- 1874 9p -do- 1r -do- 1876 6a 12a 1 LIST OF STAMPS FROM 1852 ONWARDS 1 1882-88 /2a Empire of India – Queen Victoria 9p -do- 1a -do- 1a6p -do- 2a -do- 3a -do- 4a -do- 4a6p -do- 8a -do- 12a -do- 1R -do- 1 1891 2 /2a Surcharged 1 1892-97 2 /2a 1r 1895 2r 3r 5r 1 1898 /4a 1899 3p 1900-02 3p 1 /2a 1a 2a 1 2 /2a 1902-11 3p KING EDWARD VII 1 /2a -do- 1a -do- 2a -do- 1 2 /2a -do- 3a -do- 4a -do- 6a -do- 8a -do- 12a -do- 1r -do- 2r -do- 2 LIST OF STAMPS FROM 1852 ONWARDS 3r -do- 5r -do- 10r -do- 15r -do- 25r -do- 1 1905 /4a Surcharged 1 1906 /2a Postage and Revenue 1a -do- 1911 3p KING GEORGE V 1 /2a -do- 1a -do- 1 1 /2a -do- 2a -do- 1 2 /2a -do- 3a -do- 4a -do- 6a -do- 8a -do- 12a -do- 1r -do- 2r -do- 5r -do- 10r -do- 15r -do- 25r -do- 1921 9p Surcharged 1 1922 /4a -do- 1922-26 1a Colours changed 1 1 /2a -do- 1 2 /2a -do- 3a -do- 1926-31 3p Printed at ISP Nasik 1 /2a -do- 1a -do- 1 1 /2a -do- 2a -do- 3 LIST OF STAMPS FROM 1852 ONWARDS 1 2 /2a -do- 3a -do- 4a -do- 8a -do- 12a -do- 1r -do- 2r -do- 5r -do- 10r -do- 15r -do- 25r -do- 1929 2a Air Mail Series 3a -do- 4a -

Dr. Balachandar Kathirvelu,M.B.B.S, Ph.D
Dr. Balachandar Kathirvelu, M.B.B.S, Ph.D. Clinical Assistant Professor, Rehabilitation Sciences Office: 915-747-7260 University of Texas at El Paso Email: [email protected] Office: 1101 N Campbell St. Room 306 [email protected] El Paso, Texas 79902 EDUCATION Ph.D., Tulane University, New Orleans, LA, USA Behavioral Neuroscience, 2013 Advisor: Dr. Paul J. Colombo Dissertation: Investigations of the role of the transcription factor CREB in memory formation, and interactions between the hippocampus and the striatum memory systems M.S., Tulane University, New Orleans, LA, USA Behavioral Neuroscience, 2010 Thesis: Learning-induced changes in CREB phosphorylation during memory formation: evidence for interaction between the hippocampus and dorsal striatum M.B.B.S. The Tamil Nadu Dr. M.G.R. Medical University Bachelor of Medicine, Bachelor of Surgery, 2002 Kilpauk Medical College, Chennai, TN, INDIA PROFESSIONAL APPOINTMENTS Clinical Asst Prof. University of Texas at El Paso 06/ 2019 - Present Rehabilitation Sciences Instructor of Central Michigan University, College of Medicine Anatomy 08/2018 -05/2019 Postdoctoral Central Michigan University, Neuroscience Program Research Associate 11/2017 – 05/2019 Postdoctoral Scholar The University of California, Los Angeles (UCLA) 2012 – 2017 David Geffen School of Medicine, Department of Neurology Page 1 of 6 Teaching Assistant Tulane University 2006 –2012 Neuroscience program & Department of Psychology Research Assistant Department of Public Health and Preventive medicine, Chennai, 2004 – 2005 -

Central Government Publications
March 2007 GOVERNMENT PUBLICATIONS (A) Central Government Publications 000 GENERALITIES 1 Centre for Development of Advanced Computing Annual report 2005-2006 / Centre for Development of Advanced Computing.-- Bangalor e , 200 6. 70+33p.; 28cm. In Hindi also. 004R P5 RC121584,1-4; RC121585,1-3 2 National Informatics Centre Services Inc. Annual report 2005-2006 / National Informatics Centre Services Inc.—- New Delhi, 2006. 48p.; 28cm. Bilingual. 005.16R P5 RC121605 3 Press Council of India Annual report 2005-2006 (27th) / Press Council of India.-- New Delhi, 2007. 137p.; 24cm. In Hindi also. 070.1061R P5 RC121679,1-4; RC121680,1-3 300 SOCIAL SCIENCES 4 National Scheduled Tribes Finance and Development Corporation (NSTFDC) Annual report 2005-2006 (5th) / National Scheduled Tribes Finance and Development Corporation (NSTFDC).-- New Delhi, 2006. 53+53p.; 28cm. Bilingual. 301.43R P5 RC121676,1-5 5 India. Lok Sabha (14th). Committee on Welfare of Scheduled Castes and Scheduled Tribes (2006-2007) Report of the study tour of the Committee on the welfare of scheduled castes and scheduled tribes on their visit to Mumbai, Mangalore, Mercara (COORG), Mysore and Bangalore during July 2006 / Committee on Welfare of Scheduled Castes and Scheduled Tribes (2006-2007), Lok Sabha (14th), India.-- New Delhi: Lok Sabha Secretariat, 2006. 75p.; cycl.; 28cm. In Hindi also. Chairman: Ratilal Kalidas Varma. LC 301.43R P6 RC121689-RC121690,1-5 6 India. Lok Sabha (14th). Committee on the Welfare of Scheduled Castes and Scheduled Tribes (2006-2007) 20th report on reservation for and employment of scheduled castes and scheduled Tribes in All India Institute of Medical Sciences including reservation for SCs and STs in admission therein relating to the Ministry of Health and Family Welfare (Department of Health) (Presented on March 2007) / Committee on the Welfare of Scheduled Castes and Scheduled Tribes (2006-2007), Lok Sabha (14th), India.—- New Delhi: Lok Sabha Secretariat, 2007.