Medical Research Foundation Notice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Workshop Is Designed for Following Completed Registration Form Has to Be Forwarded GGCC LLPP Workshop Professionals: to Workshop Co-Ordinator Along with Prescribed
WHO SHOULD PARTICIPATE? REGISTRATION This workshop is designed for following Completed Registration form has to be forwarded GGCC LLPP Workshop professionals: to Workshop Co-ordinator along with prescribed 18-20 June 2010 Microbiologists, Pathologists and Biochemists. Fee of Rs.3000 in the form of DD. DD should be Lab Directors / Managers and Laboratory drawn in favor of “YRG CARE” payable at Chennai. Technologists. The fee includes registration, workshop materials, QA/QC personnel, Quality Officers and Quality refreshments, breakfast and lunch provided during OVERVIEW Managers. the workshop. GCLP outline the principles and procedures to be Professionals associated with clinical The seats are restricted to 60 and participants will followed by medical laboratories involved in patient laboratory management and accreditation. be registered on “first come-first served basis” . care and/or clinical research so as to provide Only those who are currently engaged in the The registration DOES NOT cover accommodation, consistent, reproducible, auditable, and reliable diagnostic lab or clinical research are encouraged however, assistance will be provided for laboratory results; which contribute to good patient to participate in this workshop and students are arrangement of accommodation on request. not eligible. care and promote a positive attitude toward testing from a patient’s perspective. This workshop is WORKSHOP CONTENTS VENUE designed to offer comprehensive guidance for The key contents of the workshop are: those who are implementing GCLP in their TICEL Bio Park Ltd Principles of quality essentials Taramani Road, Taramani laboratories. QA/QC practices Chennai - 600113 Establishment and management of quality LEARNING OBJECTIVES ORGANIZING COMMITTEE system Documentation structure and system Organizing Chair Learn GCLP principles and their relation to Test facility operation Prof. -

Laboratories Reporting to ICMR
भारतीय आयु셍वज्ञि ान अनुसंधान पररषद वा्य अनुसंधान 셍वभाग, वा्य और पररवार क쥍याण मंत्रालय, भारत सरकार Indian Council of Medical Research Department of Health Research, Ministry of Health and Family Welfare, Government of India Date: 28/07/2021 Total Operational (initiated independent testing) Laboratories reporting to ICMR: Government laboratories : 1297 Private laboratories : 1494 - Real-Time RT PCR for COVID-19 : 1705 (Govt: 623 + Private: 1082) - TrueNat Test for COVID-19 : 938 (Govt: 625 + Private: 313) - CBNAAT Test for COVID-19 : 130 (Govt: 41 + Private: 89) - Other Molecular-Nucleic Acid (M-NA) Testing Platforms for COVID-19 : 18 (Govt: 08 + Private: 10) Note: Other Molecular-Nucleic Acid includes Abbott ID NOW, RT-LAMP, CRISPR-Cas9 and Accula™ Total No. of Labs : 2791 *CSIR/DBT/DST/DAE/ICAR/DRDO/MHRD/ISRO Laboratories. #Laboratories approved for both Real-Time RT-PCR and TrueNat/CBNAAT $Laboratories approved for both TrueNAT and CBNAAT ¥ Laboratories approved for Abbott ID NOW alone or in combination with any other testing platforms @Laboratories approved for RT-LAMP alone or in combination with any other testing platforms € Laboratories approved for CRISPR-Cas9 alone or in combination with any other testing platforms δ Laboratories approved for Accula™ alone or in combination with any other testing platforms P: Provisional Δ Pvt. Laboratories acquired by Govt. 1 | P a g e S. Test Names of States Names of Government Institutes Names of Private Institutes No. Category 1. Andhra Pradesh RT-PCR 1. Sri Venkateswara Institute of Medical 1. Manipal Hospital, Tadepalli, Guntur (131) Sciences, Tirupati 2. -

Application Form for Ug / Pg Courses the Sankara Nethralaya Academy
THE SANKARA NETHRALAYA ACADEMY (Unit of Medical Research Foundation) www.thesnacademy.ac.in City / Hospital Campus Vanagaram / Academic Campus New No.41, Old No:18, College Road, Nungambakkam, No.9, Vanagaram – Ambattur Road, Ayanambakkam, Chennai – 600 006. Ph No. 044 – 42271500 Chennai – 600 095. Ph: 044 4908 6000 E-mail: [email protected] APPLICATION FORM FOR UG / PG COURSES Registration Details: (To be filled by TSNA official) Registration Date: Registration No: Student’s Recent Remarks: Registration –in-charge (Sign) Colour Photograph Course Applied for Name of the Applicant with initials (as in +2 Mark Sheet – in BLOCK letters) Expansion of Initials Gender (Please tick) Male Female Neutral Place of Birth Date of Birth D D M M Y Y Y Y Nationality Religion Community Address for Communication Blood Group Pin Code E-mail ID Phone with STD Code Mobile No Aadhaar (UAID) No Parent Name Name of Guardian (If student not staying with parents) Parent/Guardian Address for Communication (If different from above) Pin Code E-mail ID Phone with STD Code Mobile No Details of Educational Qualifications Month & Major Name of the School / Aggregate Course Studied Year of Medium Subjects College / University % Marks /Class Passing SSLC/ 10th Std Hr. Sc. /12th Std Under Graduate Post Graduate (Enclose Attested copies of SSLC/Hr. Secondary certificates and UG Provisional Certificates or Degree Certificates.) Transfer Certificate Details (Mandatory for all courses) Certificate No Date of Issue Issuing Institution Issuing Authority Eligibility Certificate -
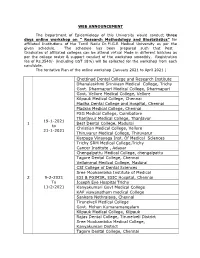
Research Methodology and Biostatistics” for Affiliated Institutions of the Tamil Nadu Dr.M.G.R Medical University As Per the Given Schedule
WEB ANNOUNCEMENT The Department of Epidemiology of this University would conduct three days online workshop on “ Research Methodology and Biostatistics” for affiliated Institutions of the Tamil Nadu Dr.M.G.R Medical University as per the given schedule. The schedule has been prepared such that Post Graduates of affiliated colleges can be attend virtual Mode in different batches as per the college roster & support conduct of the workshop smoothly. Registration fee of Rs.3540/- (including GST 18%) will be collected for the workshop from each candidate. The tentative Plan of the online workshop (January 2021 to April 2021 ) Chettinad Dental College and Research Institute Dhanalaskhmi Srinivasn Medical College, Trichy Govt. Dharmapuri Medical College, Dharmapuri Govt. Vellore Medical College, Vellore Kilpauk Medical College, Chennai Madha Dental College and Hospital, Chennai Madras Medical College, Chennai PSG Medical College, Coimbatore Thanjavur Medical College, Thanjavur 19-1-2021 1 Best Dental College, Madurai to Christian Medical College, Vellore 21-1-2021 Thiruvarur Medical College, Thiruvarur Karpaga Vinayaga Inst. Of Medical Sciences Trichy SRM Medical College,Trichy Cancer Institute , Adayar Chengalpattu Medical College, chengalpattu Tagore Dental College, Chennai Vellammal Medical College, Madurai CSI College of Dental Sciences Sree Mookambika Institute of Medical 29-2-2021 ESI & PGIMSR, ESIC Hospital, Chennai To Joseph Eye Hospital Trichy 11-2-2021 Kanyakumari Govt Medical College KAP viswanatham medical College Sankara Nethralaya, Chennai Tirunelveli Medical College Govt. Mohan Kumaramangalam Kilpauk Medical College, Kilpauk Rajas Dental College, Tirunelveli District Sree Mookambika Medical College, Kanyakumari District Tagore Dental College, Chennai Trichy SRM Medical College,Trichy Vivekanandha Dental College for Women, Namakkal Christian medical College, Vellore Govt. -

“Best Hospital in Ophthalmology” for Year 2014 Eyelights February 2015
eyelights NABL Accreditation NABH Accreditation for Sankara Nethralaya Main Number : 68 February 2015 SN confers high SN’s devout Keracon 2014 Nobility honours on its call to defy Cornea Society through 36th 3 Diabetic 4 6 7 of India’s 2nd Charity Foundation Day Retinopathy annual meet Sankara Nethralaya confers high honours on its 36th Foundation Day The Week- Nielsen survey declares Sankara Nethralaya as the “Best Hospital in Ophthalmology” for year 2014 eyelights February 2015 R e c o g n i t i o n s & A w a r d s “Best Hospital in A research paper on the molecular diagnostic tests PCRs for rapid Ophthalmology” diagnosis of tuberculosis, from Sankara Nethralaya is adjudged as for year 2014 by the best published paper in bacteriology. The Week - Nielsen The highly prestigious “The The research paper by Dr. K.Lily Therese, HOD & Sr. Prof. L&T Microbiology Week-Nielsen” survey for Research Centre, KNBIRVO was adjudged as the best paper in bacteriology rating the best hospitals in published in the Indian Journal of Medical Microbiology for year 2013, at the the country has declared XXXVIIIth National Conference of the IAMM held at SMS Medical College, Jaipur Sankara Nethralaya as the between 17th and 19th October 2014 and the presenter was honoured with a Medal. “ B e s t H o s p i t a l i n Sankara Nethralaya Academy students bag merit Ophthalmology” once again. The survey was scholarships from TechMed Healthcare, Chennai conducted on stringent Techmed healthcare organized a state level q u a l i t y m e a s u r e s b y quiz competition for the “LIFE SCIENCE experts, covering 15 cities AWARD, 2014” on 18th October, 2014. -

Global Retinoblastoma Presentation and Analysis by National Income Level
Research JAMA Oncology | Original Investigation Global Retinoblastoma Presentation and Analysis by National Income Level Global Retinoblastoma Study Group Supplemental content IMPORTANCE Early diagnosis of retinoblastoma, the most common intraocular cancer, can save both a child’s life and vision. However, anecdotal evidence suggests that many children across the world are diagnosed late. To our knowledge, the clinical presentation of retinoblastoma has never been assessed on a global scale. OBJECTIVES To report the retinoblastoma stage at diagnosis in patients across the world during a single year, to investigate associations between clinical variables and national income level, and to investigate risk factors for advanced disease at diagnosis. DESIGN, SETTING, AND PARTICIPANTS A total of 278 retinoblastoma treatment centers were recruited from June 2017 through December 2018 to participate in a cross-sectional analysis of treatment-naive patients with retinoblastoma who were diagnosed in 2017. MAIN OUTCOMES AND MEASURES Age at presentation, proportion of familial history of retinoblastoma, and tumor stage and metastasis. RESULTS The cohort included 4351 new patients from 153 countries; the median age at diagnosis was 30.5 (interquartile range, 18.3-45.9) months, and 1976 patients (45.4%) were female. Most patients (n = 3685 [84.7%]) were from low- and middle-income countries (LMICs). Globally, the most common indication for referral was leukocoria (n = 2638 [62.8%]), followed by strabismus (n = 429 [10.2%]) and proptosis (n = 309 [7.4%]). Patients from high-income countries (HICs) were diagnosed at a median age of 14.1 months, with 656 of 666 (98.5%) patients having intraocular retinoblastoma and 2 (0.3%) having metastasis. -

Sankara Nethralaya Salutes an Old Friend and Supporter on His Birth Centenary an Institution Dedicated to Service Honours a Life
eyelights NABL Accreditation NABH Accreditation for Sankara Nethralaya Main Number : 77 April 2019 Eye donation Awards SN Academy Stalwarts recall awareness conducts & greatness 2 creation 4 extensive 7 Recognitions 6 of Iravatham drive health check Mahadevan An institution dedicated to Service honours a lifelong dedication to Service Sankara Nethralaya salutes an old friend and supporter on his birth centenary eyelights April 2019 R e c o g n i t i o n s & A w a r d s Best Hospital A focussed study into the niche visual needs and challenges in Ophthalmology of the men who ensure our safety and comfort while driving / riding wins high recognition at major topical meet Dr Rashima Asokan, Assistant Professor, ESO, researcher in optometry and Member - 'Indian Association of Occupational health' was awarded the 'Golden Jubilee Medal' for her most insightful paper in the field of occupational optometry, titled 'Impact of Vision correction on the quality of life and productivity among automobile mechanics' at the 67th State meeting of the Indian Association of Occupational Health (IAOH) TN chapter. Senior Research Optometrist, Sankara Nethralaya awarded Sankara Nethralaya was with a Doctorate by SASTRA University adjudged as the 'Best Ms Kalpa Negiloni, Senior Research Optometrist, Sankara Nethralaya was H o s p i t a l i n awarded with a Doctorate by SASTRA University for her trailblazing Ophthalmology' at the finding and recommendation on the lighting and seating arrangement in 'Mayan Awards- 2018' held school classrooms, titled “Environmental factors and visual in January 2019. The award requirements in school classrooms and development of was presented by Mrs Kiran recommendation for classroom visual environment”. -

List of Board of Governors
23.09.2013 The Sankara Nethralaya Academy Board of Governors list S No Name Designation E - Mail 1 Dr A Kalanidhi Vice-Chairman – CSTAR, [email protected] Former Vice Chancellor, Anna University, Chennai 2 Dr Ashok Jhunjhunwala Chairman & Professor - Electrical Engineering [email protected] Department, IIT Madras 3 Dr Lingam Gopal Board Member, Medical Research Foundation, [email protected] Chennai 4 Dr Neil Miller Professor of Ophthalmology, Neurology, and [email protected] Neurosurgery at the Johns Hopkins Medical Institutions 5 Dr Ravindra D Bapat Former Vice-Chancellor of Mahatma Gandhi [email protected] Mission University of Health Sciences 6 Dr S Bhaskaran Chairman,Medical Research Foundation, [email protected] Chennai 7 Dr S Swaminathan Director, Centre for Nanotechnology & [email protected] Advanced Biomaterials.SASTRA University,Thanjavur 8 Dr T K Parthasarathy Pro Chancellor -Sri Ramachandra [email protected] University,Chennai 9 Dr Vasan K S Managing Director, Medical Research [email protected] Foundation, Chennai 10 Dr Vijay Dasmania Vice Chancellor – HIHT University,(The [email protected] Himalayan Institute Hospital Trust), Dehradun, Uttaranchal 11 Dr S Meenakshi Director – Academics, Medical Research [email protected] Foundation, Chennai 12 Dr. S. S. Badrinath Chairman Emeritus & President, Medical [email protected] Research Foundation, Chennai 13 Lion Dr Chandrasekara Vice Chancellor-Sri Devaraj Urs Academy of [email protected] Shetty Higher Education and Research, Karnataka 14 Lion P Haridas Senior Advocate & Secretary [email protected] D G Vaishnav College, Chennai 15 Mr D R Karthikeyan Advisor: Law-Human Responsibilities- [email protected] Corporate Affairs 16 Mr Homi Bhabha Director – Mercl Ltd,& Ceekay Daikin Ltd [email protected] 17 Mr MS Jayaraman Management Consultant & Coach (Leadership [email protected] building), Atheros, Chennai. -

2016-2017 Indian Institute of Technology Madras
Indian Institute of Technology Madras 2016-2017 No. 1 Engineering Institute in the Country for 2016, 2017 & 2018 As per National Institutional Ranking Framework, MHRD, Govt. of India CoNtents YEAR AT A GLANCE 2 DIRECTOR’S REPORT 4 ADMINISTRATION 20 ACADEMIC PROGRAMMES AND AWARD OF DEGREES 24 DEPARTMENTS CENTRES OF SPECIAL FACILITIES DEPARTMENT OF AEROSPACE ENGINEERING 34 CENTRE FOR INDUSTRIAL CONSULTANCY & SPONSORED RESEARCH 68 DEPARTMENT OF APPLIED MECHANICS 36 CENTRE FOR CONTINUING EDUCATION 70 DEPARTMENT OF BIOTECHNOLOgy 38 P.G. SENAPATHY CENTRE FOR COMPUTING RESOURCES 71 DEPARTMENT OF CHEMISTRY 40 CENTRAL ELECTRONICS CENTRE 72 DEPARTMENT OF CHEMICAL ENGINEERING 42 SOPHISTICATED ANALYTICAL INSTRUMENT FACILITY 73 DEPARTMENT OF CIVIL ENGINEERING 44 CENTRAL FACILITIES 74 DEPARTMENT OF COMPUTER SCIENCE AND ENGINEERING 46 CENTRAL LIBRARY 75 DEPARTMENT OF ELECTRICAL ENGINEERING 48 STUDENTS AMENITIES & ACTIVITIES 76 DEPARTMENT OF ENGINEERING DESIGN 50 INTERNATIONAL & ALUMNI RELATIONS 78 DEPARTMENT OF HUMANITIES AND SOCIAL SCIENCES 52 CENTRES OF EXCELLENCE 80 DEPARTMENT OF MANAGEMENT STUDIES 54 STUDENTS PLACEMENT 81 DEPARTMENT OF MATHEMATICS 56 FINANCIAL ASSISTANCE TO STUDENTS 82 DEPARTMENT OF MECHANICAL ENGINEERING 58 FINANCE & ACCOUNTS 84 DEPARTMENT OF METALLURGICAL AND MATERIALS ENGINEERING 60 CAMPUS AMENITIES 86 DEPARTMENT OF OCEAN ENGINEERING 62 DEPARTMENT OF PHYSICS 64 Year Book 2016–17 1 YEAR At A GlANCE UG Students on roll 1982 465 UG Admissions PG Students on roll 4431 1316 PG Admissions Research Scholars on roll 2767 494 Research -

Chennai PPN Network Hospital List Sr
Chennai PPN Network Hospital List Sr. Tel_are Hospital Name Location Address Pin_No Tel_No MobileNo City State E_Mail PPN City No. a_code 81-86 Annai Valasaravakk Tamil [email protected] 1 A N N Hospital Therasa Street 600087 044 24869300 9442360800 Chennai Chennai am Nadu om Indira Nagar # 172, SOLAIAPPAN STREET, NEAR drselvaraj@avhospital Tamil 2 A V Hospitals Parrys MAHARANI 600021 044 25955859 9444013879 Chennai s.com;kalaivani.vetrise Chennai Nadu THEATRE, [email protected] MANNADY [email protected] No. 395, T H Road, Tamil m;aakashsrk_dr@yah 3 Aakash Hospital Thiruvotriyur Near Thiruvottriyur 600019 044 25730099 9444382293 Chennai Chennai Nadu oo.co.in;aakashsrkdr Bus Terminus @gmail.com abhijay.claims@gmail. 22/2, E.S.I Hospital Abhijay Hospital (p) Tamil com; 4 Perambur Road, Perambur, 600011 044 49015050 9884368589 Chennai Chennai Ltd Nadu cashless@abhijayhosp Peravallur itals.com adityahospital@gmail. 7, Barnaby Road, Tamil com; 5 Aditya Hospital Kilpauk 600010 044 26411447 9840727909 Chennai Chennai Kilpauk, Chennai Nadu insurance@adityahos pital.co.in insurance@agadaheal thcare.net;karthikaran No 8, Dr Nair Road, i.p@agadahealthcare. Agada Healt Care Tamil 6 T Nagar Behind Vani Mahal, 600017 044 28152604 9087718512 Chennai net;prabhub@agadah Chennai Pvt Ltd Nadu T Nagar ealthcare.net; operations@agadahea lthcare.net 1,Sowrastra Nungambakk Tamil sureshdrsuresh@yaho 7 Amma Hospital Nagar,7th 600094 044 24840441 9840048896 Chennai Chennai am Nadu o.co.in; Street,Choolaimedu new no 80 ,7 th ammayieyehospital@y Ammayi Eye Tamil -

CTRI Trial Data
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Wed, 29 Sep 2021 03:01:46 GMT) CTRI Number CTRI/2009/091/000298 [Registered on: 16/07/2009] - Last Modified On 26/09/2014 Post Graduate Thesis No Type of Trial Interventional Type of Study Vaccine Other (Specify) [Pilot study to study the safety and immunogenicity of Merck Quadrivalent HPV vaccine] Study Design Single Arm Trial Public Title of Study A clinical trial to study prevention of HPV in HIV-Positive women in India Scientific Title of A Single-Arm, Open-Label, Pilot Study of the Safety and Immunogenicity of the Merck Quadrivalent Study Human Papillomavirus Vaccine among HIV-Positive Women in India: A Trial of AIDS Malignancy Clinical Trials Consortium Acronym: AMC Protocol # 054 Secondary IDs if Any Secondary ID Identifier AMC-054 Version 9.0; May 4 2012 Protocol Number NCT00667563 ClinicalTrials.gov Details of Principal Details of Principal Investigator Investigator or overall Name Dr N Kumarasamy Trial Coordinator (multi-center study) Designation Principal Investigator Affiliation YR Gaitonde Medical Educational & Research Foundation Address YR Gaitonde Medical Educational & Research Foundation, Voluntary Health Services, Rajiv Gandhi Salai, Taramani Chennai TAMIL NADU 600113 India Phone 04439106789 Fax 04422542949 Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr N Kumarasamy Query) Designation Principal Investigator Affiliation YR Gaitonde Medical Educational & Research Foundation Address YR Gaitonde Medical Educational & Research Foundation, Voluntary Health Services, Rajiv Gandhi Salai, Taramani Chennai TAMIL NADU 600113 India Phone 04439106789 Fax 04422542949 Email [email protected] Details Contact Details Contact Person (Public Query) Person (Public Query) Name Sumeela Nair Designation Lead Data Manager Affiliation The EMMES Corporation Address EMMES Services Pvt. -

Directory of Services
SRI NATHELLA SAMPATHU CHETTY CLINICAL LABORATORY (UNIT OF MEDICAL RESEARCH FOUNDATION) 2018 DIRECTORY OF SERVICES DIRECTORY OF SERVICES SRI NATHELLA SAMPATHU CHETTY CLINICAL LABORATORY (UNIT OF MEDICAL RESEARCH FOUNDATION) No.41, College Road, Chennai – 600 006 Ph: 2823 3556 / 2827 1616 / 2831 1913 Fax: 91-044-2825 4180 Email: [email protected] Issue No : 1 Issue Date : 26.07.2018 Amend No : 0 Page 1 of 88 Prepared & Issued by: Approved by: Quality Manager Management Representative Dr.N.Angaarkanni Ph.D Dr.SB.Vasanthi MBBS SRI NATHELLA SAMPATHU CHETTY CLINICAL LABORATORY (UNIT OF MEDICAL RESEARCH FOUNDATION) 2018 DIRECTORY OF SERVICES TABLE OF CONTENTS S.No CONTENTS Page No. 1. Title Page 1 - 1 2. Table of Contents 2 - 3 3. General Information of Laboratory Services 4 - 6 4. Contact Details 7 - 9 5. Quality Policy & Quality Objectives 10 – 10 6. Laboratory Charges 11 - 18 7. Package Tests 19 - 23 8. General Instruction on Sample Collection 24 – 27 9. DEPARTMENT OF HAEMATOLOGY & CLINICAL PATHOLOGY 28 – 33 Test Master List / Turn Around Time Acceptance and Rejection Criteria for 34 – 35 Collection Area, Hematology & Clinical Pathology 10. DEPARTMENT OF CLINICAL & SPECIAL BIOCHEMISTRY 36 – 42 Test Master List / Turn Around Time Acceptance and Rejection Criteria 43 – 45 11. DEPARTMENT OF MICROBIOLOGY & SEROLOGY 46 – 62 Test Master List / Turn Around Time Acceptance and Rejection Criteria 63 – 64 Issue No : 1 Issue Date : 26.07.2018 Amend No : 0 Page 2 of 88 Prepared & Issued by: Approved by: Quality Manager Management Representative Dr.N.Angaarkanni Ph.D Dr.SB.Vasanthi MBBS SRI NATHELLA SAMPATHU CHETTY CLINICAL LABORATORY (UNIT OF MEDICAL RESEARCH FOUNDATION) 2018 DIRECTORY OF SERVICES 12.