CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Retinoblastoma Presentation and Analysis by National Income Level
Research JAMA Oncology | Original Investigation Global Retinoblastoma Presentation and Analysis by National Income Level Global Retinoblastoma Study Group Supplemental content IMPORTANCE Early diagnosis of retinoblastoma, the most common intraocular cancer, can save both a child’s life and vision. However, anecdotal evidence suggests that many children across the world are diagnosed late. To our knowledge, the clinical presentation of retinoblastoma has never been assessed on a global scale. OBJECTIVES To report the retinoblastoma stage at diagnosis in patients across the world during a single year, to investigate associations between clinical variables and national income level, and to investigate risk factors for advanced disease at diagnosis. DESIGN, SETTING, AND PARTICIPANTS A total of 278 retinoblastoma treatment centers were recruited from June 2017 through December 2018 to participate in a cross-sectional analysis of treatment-naive patients with retinoblastoma who were diagnosed in 2017. MAIN OUTCOMES AND MEASURES Age at presentation, proportion of familial history of retinoblastoma, and tumor stage and metastasis. RESULTS The cohort included 4351 new patients from 153 countries; the median age at diagnosis was 30.5 (interquartile range, 18.3-45.9) months, and 1976 patients (45.4%) were female. Most patients (n = 3685 [84.7%]) were from low- and middle-income countries (LMICs). Globally, the most common indication for referral was leukocoria (n = 2638 [62.8%]), followed by strabismus (n = 429 [10.2%]) and proptosis (n = 309 [7.4%]). Patients from high-income countries (HICs) were diagnosed at a median age of 14.1 months, with 656 of 666 (98.5%) patients having intraocular retinoblastoma and 2 (0.3%) having metastasis. -

2016-2017 Indian Institute of Technology Madras
Indian Institute of Technology Madras 2016-2017 No. 1 Engineering Institute in the Country for 2016, 2017 & 2018 As per National Institutional Ranking Framework, MHRD, Govt. of India CoNtents YEAR AT A GLANCE 2 DIRECTOR’S REPORT 4 ADMINISTRATION 20 ACADEMIC PROGRAMMES AND AWARD OF DEGREES 24 DEPARTMENTS CENTRES OF SPECIAL FACILITIES DEPARTMENT OF AEROSPACE ENGINEERING 34 CENTRE FOR INDUSTRIAL CONSULTANCY & SPONSORED RESEARCH 68 DEPARTMENT OF APPLIED MECHANICS 36 CENTRE FOR CONTINUING EDUCATION 70 DEPARTMENT OF BIOTECHNOLOgy 38 P.G. SENAPATHY CENTRE FOR COMPUTING RESOURCES 71 DEPARTMENT OF CHEMISTRY 40 CENTRAL ELECTRONICS CENTRE 72 DEPARTMENT OF CHEMICAL ENGINEERING 42 SOPHISTICATED ANALYTICAL INSTRUMENT FACILITY 73 DEPARTMENT OF CIVIL ENGINEERING 44 CENTRAL FACILITIES 74 DEPARTMENT OF COMPUTER SCIENCE AND ENGINEERING 46 CENTRAL LIBRARY 75 DEPARTMENT OF ELECTRICAL ENGINEERING 48 STUDENTS AMENITIES & ACTIVITIES 76 DEPARTMENT OF ENGINEERING DESIGN 50 INTERNATIONAL & ALUMNI RELATIONS 78 DEPARTMENT OF HUMANITIES AND SOCIAL SCIENCES 52 CENTRES OF EXCELLENCE 80 DEPARTMENT OF MANAGEMENT STUDIES 54 STUDENTS PLACEMENT 81 DEPARTMENT OF MATHEMATICS 56 FINANCIAL ASSISTANCE TO STUDENTS 82 DEPARTMENT OF MECHANICAL ENGINEERING 58 FINANCE & ACCOUNTS 84 DEPARTMENT OF METALLURGICAL AND MATERIALS ENGINEERING 60 CAMPUS AMENITIES 86 DEPARTMENT OF OCEAN ENGINEERING 62 DEPARTMENT OF PHYSICS 64 Year Book 2016–17 1 YEAR At A GlANCE UG Students on roll 1982 465 UG Admissions PG Students on roll 4431 1316 PG Admissions Research Scholars on roll 2767 494 Research -

Narrative Notes on Plan Programmes Tam-N
y » I ; ^ t O M i T*' NARRATIVE NOTES ON PLAN PROGRAMMES ANNUAL PLAN 2000-01 STATE PLANNING COMMISSION CHENNAI - 600 005 - 5 * 4 8 2 3 0 < » - 2 5 - TAM-N AUGUST 2000 NARRATIVE NOTES ON PLAN PROGRAMMES 2000-01 NIEPA DC D11079 ' xA^\Q§ i , , .‘♦1 Zi. i-I. Mr:,-, ' 3 )-u o 79 V ^ ' ' Z4* - o 4"* Zc © I CONTENTS Page 1. Crop Husbandry 1 2. Research and Education 25 3. Food, Storage & WareHousing 30 4. Soil & Water Conservation 35 5. Animal Husbandry 41 6. Dairy Developnnent 50 7. Fisheries 53 8. Forests 61 9. Investment in Agri.Financial Institutions 69 10. Co-operation 71 11. Special Programme for Rural Development 75 12. Land Reforms 79 13. Community Development 80 14. Minor Irrigation 83 15. Command Area Development 88 16. Major, Medium Irrigation & Flood Control 90 17. Power Development 103 18. Non-Conventional Sources of Energy 111 19. Industries- Medium and Large 114 20. Village and Small Industries 130 21. Weights and Measures 142 22. Mining and Metallurgical Industries 143 23. Roads and Bridges 145 24. Road and Inland Water Transport 156 25. Scientific Services and Research 158 26. Ecology and Environment 163 27. Secretariat Economic Services 166 28. Tourism 171 29. Economic Advice and Statistics 175 30. Civil Supplies 179 31. General Education 184 CONTENTS—conf. Pagee 32. Technical Education 1988 33. Art and Culture 2011 34. Sports and Youth Services 207)7 35. Medical 21C0 36. Public Health 2188 37. Water Supply and Sanitation 2332 38. Housing 24ft6 39. Urban Development 2551 40. Information and Publicity 2558 41. -

Central Leather Research Institute, Adyar, Chennai- 600020
CENTRAL LEATHER RESEARCH INSTITUTE, ADYAR, CHENNAI- 600020 LIST OF APPROVED HOSPITALS AND ITS BRANCHES FOR HEALTH SERVICES BY CLRI AS ON 01-04-2019 REIMBURSEMENT AS PER CGHS RATES ONLY Sl. No Name of the Hospitals Address 1. Amrit Hospital Mint Street, Sowcarpet, Chennai - 79 2. Adyar P.M. Hospital & Research Centre Pvt. Ltd Lattice Bridge Rd, Adyar, Chennai - 20 3. Andhra Mahila Sabha R.A.Puram, Chennai - 28 4. Apollo Speciality Hospital – OMR Perungudi, Chennai - 96 5. Apollo Hospitals Enterprises Greams Road, Chennai - 6 6. Apollo Multispeciality Hospitals Teynampet, Chennai - 18 7. Appasamy Medicare Centre (P) Ltd Arumbakkam, Chennai - 106 8. Aswine Soundra Hospital and Research Centre Teynampet, Chennai - 18 9. Athipathy Hospital & Research Centre Velachery, Chennai - 42 10. Balaji Hospitals Private Ltd Guindy, Chennai - 32 11. Balakrishna Eye Hospital & Eye Research Centre Saidapet, Chennai - 15 12. Barathiraja Hospital and Research Centre Pvt. T.Nagar, Chennai - 17 Ltd 13. Beach Chennai Hospital New Washermanpet, Chennai - 81 14. Billroth Hospital Shenoy Nagar, Chennai - 30 15. BSS Hospital Mandaveli, Chennai - 28 16. Cancer Institute (WIA) Adyar, Chennai - 20 17. Chettinad Health City Kelambakkam, Chennai-603103 18. Child Trust Hospital Nungambakkam, Chennai - 34 19. Al-Saudi Clinical Services (Citi Hospital) Adyar, Chennai - 20 20. CSI Kalyani General Hospital Dr. R.K.Salai, Chennai - 4 21. CSI Rainy Multi Speciality Hospital 45, GA Road, Chennai - 21 22. Deepam Hospitals (P) Ltd West Tambaram, Chennai - 45 23. Chennai Meenakshi Multispeciality Hospital Mylapore, Chennai - 4 24. Chennai National Hospital Beach Lane, Chennai - 1 25. Dr. Agarwal Eye Hospital Cathedral Road, Chennai - 86 26. Dr. A. -

Medical Research Foundation Notice
MEDICAL RESEARCH FOUNDATION To The Members of the Medical Research Foundation 7th September 2011 NOTICE NOTICE IS HEREBY GIVEN TO THE MEMBERS OF THE MEDICAL RESEARCH FOUNDATION THAT THE 33RD ANNUAL GENERAL MEETING OF THE FOUNDATION WILL BE HELD ON THRUSDAY, THE 29th SEPTEMBER 2011 AT 6.00 P.M. AT BOARD ROOM, 2ND FLOOR, KAMALNAYAN BAJAJ RESEARCH CENTRE BLOCK, NEW NO 41, OLD NO 18, COLLEGE ROAD, CHENNAI 600 006. THE AGENDA FOR THE MEETING IS GIVEN BELOW: AGENDA 1. To consider and adopt the Annual report of the Foundation for the year 2010-11. 2. To consider and adopt the Audited Income and Expenditure Account for the year ended March 31, 2011 and the Balance Sheet as at that date together with the Report of the Auditors thereon. 3. To appoint Auditors and to fix their remuneration for the year ending 31st March 2012. 4. To elect Members to the Board of Management in the vacancies caused by the retirement of the following Members, who are eligible for re-appointment. Name Category Mr. H D Malesra Patron Member Mr.Mani S Subramonian Patron Member 5. To elect Members to the Board of Management in the existing vacancy. Kindly make it convenient to attend the meeting. N.SUGALCHAND JAIN HONY. SECRETARY & TREASURER 1 MEDICAL RESEARCH FOUNDATION SANKARA NETHRALAYA MISSION STATEMENT QUALITY OBJECTIVES The mission of Sankara Nethralaya is to To maintain the quality of ophthalmic provide Total Eye-care solutions of highest s e r v i c e s i n a c c o r d a n c e w i t h standards to all sections of community international standards. -
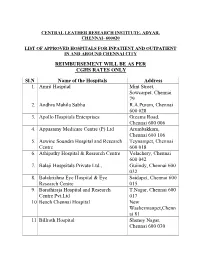
REIMBURSEMENT WILL BE AS PER CGHS RATES ONLY Sl.N Name Of
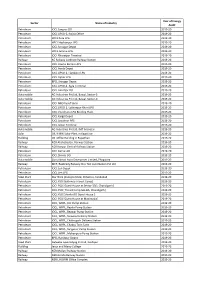
CENTRAL LEATHER RESEARCH INSTITUTE, ADYAR, CHENNAI- 600020 LIST OF APPROVED HOSPITALS FOR INPATIENT AND OUTPATIENT IN AND AROUND CHENNAI CITY REIMBURSEMENT WILL BE AS PER CGHS RATES ONLY Sl.N Name of the Hospitals Address 1. Amrit Hospital Mint Street, Sowcarpet, Chennai 79 2. Andhra Mahila Sabha R.A.Puram, Chennai 600 028 3. Apollo Hospitals Enterprises Greams Road, Chennai 600 006 4. Appasamy Medicare Centre (P) Ltd Arumbakkam, Chennai 600 106 5. Aswine Soundra Hospital and Research Teynampet, Chennai Centre 600 018 6. Athipathy Hospital & Research Centre Velachery, Chennai 600 042 7. Balaji Hospsitals Private Ltd., Guiindy, Chennai 600 032 8. Balakrishna Eye Hospital & Eye Saidapet, Chennai 600 Research Centre 015 9. Barathiraja Hospital and Research T.Nagar, Chennai 600 Centre Pvt.Ltd 017 10. Beach Chennai Hospital New Washermanpet,Chenn ai 81 11. Billroth Hospital Shenoy Nagar, Chennai 600 030 12. BSS Hospital Mandaveli, Chennai 600 028 13. Cancer Institute (WIA) Adyar, Chennai 600 020 14. Chettinad Health City Kelambakkam, Chennai 15. Child Trust Hospital Nungambakkam, 600 034 16. City Hospital Adyar, Chennai 600 020 17. CSI Kalyani General Hospital Dr. R.K.Salai, Chennai 600 004 18. CSI Rainy Multi Speciality Hospital 45 GA Road, Chennai 600 021 19. Deepam Hospitals (P) Ltd., West Tambaram, Chennai 600 045 20. Devaki Hospital Ltd Mylapore, Chennai 600 004 21. Dr. Agarwa l Eye Hospital Cathedral Road, Chennai 600 086 22. Dr. Meenakshi Hospitals Avadi, Chennai 600 054 23. Dr. Rabindran’s Health Care Centre Pvt. Ambattur, Chennai Ltd., 600 053 24. Dr. Rai Memorial Medical Centre Anna Salai, Chennai 600 018 25. -

(WIA) COLLEGE of ONCOLOGICAL SCIENCES Adyar, Chennai 600
CANCER INSTITUTE (WIA) Signed photograph to COLLEGE OF ONCOLOGICAL SCIENCES be pasted Adyar, Chennai 600 020 APPLICATION FOR ADMISSION TO MD (RADIOTHERAPY) / DMRT / BOTH MD & DMRT COURSE(S) - (2017-18 SESSION) 1 Name in BLOCK LETTERS with expanded initials at the end. Women candidates should add Selvi / Tmt. as the case may be. Name of the candidate should be underlined. 2 a) Complete address to which any communication is to be sent. b) Telephone no. (with code), if any c) Mobile No., if any d) E-mail id, if any. e) Fax no. (with code), if any. 3 Have you applied for any other course? If so, give details & order of priority. 4 Have you applied for any job? If so, give details & order of priority. 5 Nationality. (Evidence to be produced) 6 a) Present occupation, if any. b) Official address. c) Are you a deputed candidate? (If so, the necessary communication should be enclosed. If not a deputed candidate, a ‘No Objection Certificate’ from the employer should be enclosed) 7 Name, occupation & address of father / mother / husband / wife. 8 Do you belong to the scheduled caste / schedule tribe / socially & educationally backward classes specified in the Tamil Nadu Education Rules? (Evidence to be produced) 9 Date of birth & Age. (Evidence to be produced) 10 Mother tongue. 11 Medical qualification(s). 12 MBBS degree: a) College from which qualified. b) University from which qualified. c) Whether the University is recognized by Medical Council of India / the Tamil Nadu Dr MGR Medical University. d) Register number of the qualifying examination. e) Month & year of passing. -

Nin/PMJAY ID Name of the Vaccination Site* Category* Type* District* Block* ABHIJAY HOSPITAL PERAMBUR, Chennaiprivate TN
NiN/PMJAY ID Name of the Vaccination Site* Category* Type* District* Block* ABHIJAY HOSPITAL PERAMBUR, CHENNAIPrivate TN. Paid CHENNAI Chennai National Hospital, Chennai TN.Private Paid Chennai Life Care Hospital, Chennai TN. Private Paid Chennai SAMPAT NURSING HOME,CHENNAI TN.Private Paid Chennai laksha Mylapore,Chennai TN. Private Paid Chennai Trinity Acute care,chennai TN. Private Paid Chennai CSI KALYANI GENERAL HOSPITAL, ChennaiPrivate TN. Paid Chennai Vivekananda hospital,Mylapore,ChennaiPrivate TN. Paid Chennai Kavery Hospital Chennai,TN. Private Paid Chennai Laksha Hospital,Chennai TN. Private Paid Chennai Sakthi Hospital, Triplicane, Chennai TN.Private Paid Chennai Apollo Childrens Hospital,Chennai TN.Private Paid Chennai Apollo Greams,Chennai TN. Private Paid Chennai Medical Research Foundation,ChennaiPrivate TN. Paid Chennai Chennai City Police Hospital,Chennai Private Paid Chennai Apollo First Med Kilpauk,Chennai TN.Private Paid Chennai The Guest Hospital, Chennai TN. Private Paid Chennai Kumaran Hospital Kilpauk, Chennai TN.Private Paid Chennai lswarya Women Kilpauk,Chennai TN. Private Paid Chennai Tosh Hospital,Chennai TN. Private Paid Chennai Aysha Hospital Kilpauk Chennai TN. Private Paid Chennai Lifeline Multispeciality Hospital, ChennaiPrivate TN. Paid Chennai Murugan Hospital kilpauk, Chennai TN.Private Paid Chennai Ramarau Polyclinic,Kilpauk,Chennai TN.Private Paid Chennai New Hope Medical Center, Chennai TN.Private Paid Chennai KKR ENT Hospital And Institute, ChennaiPrivate TN. Paid Chennai Sen Hospital Perambur,Chennai TN. Private Paid Chennai Srinivas Priya Hospital Chennai TN. Private Paid Chennai S.G Hospital, Chennai TN. Private Paid Chennai Muthu Hospital,Chennai TN. Private Paid Chennai Sudha Hospital, Chennai TN. Private Paid Chennai Sri Kumaran Multi Speciality Hospital,chennaiPrivate TN.Paid Chennai Shanmugam Multi Speciality Hospital,Private Chennai Paid Chennai Nichani Hospital Royapuram Chennai PrivateTN. -

Hospital Name Address
SLNO ZONE WARD HOSPITAL NAME ADDRESS 1 01-Thiruvottyur 001 SUMITHIRA CLINIC K.H. ROAD KATHIVAKKAM THALANKUPPAM 600057 2 01-Thiruvottyur 001 URBAN HEALTHPOST KAMARAJ NAGAR KATHIVAKKAM KAMARAJ NAGAR 600057 3 01-Thiruvottyur 001 SRI VEGNESH CLINIC C-15,THLANKUPPAM MAIN ROAD KATHIVAKKAM ULAGANATHAPURAM 600057 4 01-Thiruvottyur 001 S.S. CLINIC 6,6TH STREET KATHIVAKKAM ULAGANATHAPURAM 600057 5 01-Thiruvottyur 001 JAI CLINIC 3,7TH STREET ULAGANATHAPURAM THALANKUPPAM 600057 6 01-Thiruvottyur 001 JAI ASHWIN CLINIC 4, SATHYAVANI MUTHU NAGAR 7TH STREET, ENNORE, CHENNAI 600057 7 01-Thiruvottyur 001 SRI VEGNESH CLINIC C-15,THALANKUPPAM MAIN ROAD KATHIVAKKAM ULAGANATHAPURAM 600057 8 01-Thiruvottyur 002 RAJA CLINIC GIRIYAPPATHOTTAM KATHIVAKKAM SIVANPADAI VEEDHI 600057 9 01-Thiruvottyur 002 SRI VENUGOPAL CLINIC R.S. ROAD KATHIVAKKAM VALLUVAR NAGAR 600057 10 01-Thiruvottyur 002 SRI VENKATESWARA CLINIC K.H. ROAD KATHIVAKKAM SANJAIGANDHI NAGAR 600057 11 01-Thiruvottyur 002 THANGAMMAL CLINIC K.H. ROAD KATHIVAKKAM KATTUKUPPAM 600057 12 01-Thiruvottyur 002 MATERNITY CENTRE VILLAGE STREET KATHIVAKKAM SIVANPADAI VEEDHI 600057 13 01-Thiruvottyur 002 VIJAYA CLINIC THLANKUPPAM KATHIVAKKAM THALANKUPPAM 600057 14 01-Thiruvottyur 002 IPP-V HOSPITAL 03/592,K.H. ROAD KATHIVAKKAM K.H.ROAD 600057 15 01-Thiruvottyur 002 IPP-V HOSPITAL KAMARAJ NAGAR 5TH STREET KATHIVAKKAM KAMARAJ NAGAR 600057 16 01-Thiruvottyur 002 VIJAYA CLINIC GANDHI NAGAR KATHIVAKKAM GANDHI NAGAR 600057 17 01-Thiruvottyur 004 SELVA CLINIC & MATERNITY CENTR 72,KAMARAJ NAGAR THIRUVOTTIYUR ERNAVOOR 600019 -
List of Energy Audits for PCRA Portal Upload.Xlsx
Year of Energy Sector Name of Industry Audit Petroleum IOCL Sangrur DO 2019-20 Petroleum IOCL UPSO-2, Noida Office 2019-20 Petroleum HPCL Kota LPG 2019-20 Petroleum HPCL Hoshiarpur LPG 2019-20 Petroleum IOCL Srinagar Depot 2019-20 Petroleum HPCL Jammu LPG 2019-20 Petroleum IOCL Bharatpur Terminal 2019-20 Railway NE Railway Lucknow Railway Station 2019-20 Petroleum IOCL Utarlai Barmer AFS 2019-20 Petroleum IOCL Aonla Depot 2019-20 Petroleum IOCL UPSO-2, Haridwar LPG 2019-20 Petroleum IOCL Ajmer LPG 2019-20 Petroleum BPCL Srinagar Depot 2019-20 Petroleum IOCL UPSO-2, Agra Terminal 2019-20 Petroleum IOCL Amritsar DO 2019-20 Automobile AG Industries Pvt Ltd, Bawal, Sector-5 2019-20 Automobile AG Industries Pvt Ltd, Bawal, Sector-6 2019-20 Petroleum IOCL NRO Yusuf Sarai 2019-20 Petroleum IOCL UPSO-2, Lakhimpur Kheri LPG 2019-20 Petroleum IOCL Jhunjhunu LPG Bottling Plant 2019-20 Petroleum IOCL Kargil Depot 2019-20 Petroleum IOCL Jaisalmer AFS 2019-20 Petroleum IOCL Jaipur Terminal 2019-20 Automobile AG Industries Pvt Ltd, IMT Manesar 2019-20 Solar OIL 9 MW Solar Plant in Rajasthan 2019-20 Building OIL Office Building in Rajasthan 2019-20 Railway NCR Allahabad Jn. Railway Station 2019-20 Railway NCR Kanpur Central Railway Station 2019-20 Petroleum IOCL Karnal AO 2019-20 Petroleum IOCL Shimla DO 2019-20 Automobile Guru Nanak Auto Enterprises Limited, Phagwara 2019-20 Railway MCF, Raebreily Railway thru TAC Automation Pvt Ltd 2019-20 Petroleum IOCL Leh Depot 2019-20 Petroleum IOCL Leh LPG 2019-20 Steel Plant Star Wire (India) Limited, Chhainsa, -

2006-2014 Annual Report
www.yrgcare.org www.ecokitchen.org YR Gaitonde Medical, Educational and Research Foundation 2006 - 2014 2006-2014 Contents The YR Gaitonde Medical, Educational And Research Foundation 02 Note from the Managing Trustee 03 Legal Information 04 Institutional Review Board 07 Community Advisory Board 09 Human Resources 11 Y R Gaitonde Centre For AIDS Research And Education [YRGCARE] 12 Awards, Honors and Recognitions 13 Hospital 16 Camp Rainbow 27 Infectious Diseases Laboratory 34 Community Outreach 44 Training and Symposiums 49 Enhancing Community Opportunities [ECO] 60 Pibags 63 ECO Kitchen 65 Endrum Punnagai 76 Strategic Partnerships 79 Journal Publications 83 Research 103 Summary of Financial Statements 129 1 2006-2014 The YR Gaitonde Medical, Educational and Research Foundation The Y.R. Gaitonde Medical, Educational and Research Foundation, honours the memory of Late Shri Yeshwant Rao Gaitonde. Founded in 1985, the Foundation pioneers public health initiatives through the YR Gaitonde Centre for AIDS Research and Education (YRGCARE), and champions livelihood and food security initiatives through ECO Kitchen and Pibags. Since 1993 Since 2009 2 2006-2014 Note from the Managing Trustee I am pleased to present a review of our activities from Project” provided critical treatment, peer support, and for women in need. These projects bring opportunities April 2006 until March 2014. economic assistance to women affected by HIV in the to those whom we have proudly served in the last two five South Indian states. YRGCARE introduced free decades: women facing partner violence, spouses of In recognition that the HIV epidemic disproportionately nutrition support for patients during their in patient persons who inject drugs and other socially vulnerable affects women, YRGCARE devoted significant resources care. -

Trade Marks Journal No: 1975 , 23/11/2020 Class 34
Trade Marks Journal No: 1975 , 23/11/2020 Class 34 3918558 17/08/2018 VAIBHAV BAHARIA KOTHAR MOHALA,SHAHPURA(BHILWARA)RAJ, BHILWARA-311404 The Trade Marks Act, 1999 Address for service in India/Attorney address: VANCHINATHAN No 6, Dhanammal Street Spurtank Road Chetpet Chennai - 600031 Proposed to be Used AHMEDABAD Tobacco, smokers’ articles, matches. 3854 Trade Marks Journal No: 1975 , 23/11/2020 Class 34 3970002 10/10/2018 NIRMAL JOSHI, TRADING AS:- APNA PRODUCTS GOPALNAGAR, CHITTORGARH – 312 001 (RAJASTHAN) INDIA A Proprietorship Firm Address for service in India/Attorney address: TRADESAFE GHANSHYAM HOUSE, BUNGLOW NO. 9, SHRINAGAR SOCIETY, OPP. SARDAR PATEL STADIUM, NEAR GOLDEN TRIANGLE, AHMEDABAD – 380 014 (GUJARAT) INDIA Used Since :27/08/1984 AHMEDABAD TOBACCO, UNMANUFACTURED TOBACCO, READY TO EAT FILTER TOBACCO, LIME MIX CHEWING TOBACCO, ZARDA. .. THIS IS SUBJECT TO ASSOCIATION WITH REGISTERED/PENDING REGISTRATION NO..3970003, 3970004, 1284067.. 3855 Trade Marks Journal No: 1975 , 23/11/2020 Class 34 4001997 19/11/2018 DS INNOVATIVE PRODUCTS LLP 4828/24, plot no. 2 G/F Basement, Ward no. XI, Daryaganj, Delhi 110002 A COMPANY INCORPORATED UNDER THE INDIAN COMPANIES ACT Address for service in India/Agents address: THE ACME COMPANY B-41, NIZAMUDDIN EAST, NEW DELHI - 110013. Proposed to be Used DELHI Tobacco; smokers’ articles; matches, lighters for smokers 3856 Trade Marks Journal No: 1975 , 23/11/2020 Class 34 4001999 19/11/2018 DS INNOVATIVE PRODUCTS LLP 4828/24, plot no. 2 G/F Basement, Ward no. XI, Daryaganj, Delhi 110002 A COMPANY INCORPORATED UNDER THE INDIAN COMPANIES ACT Address for service in India/Agents address: THE ACME COMPANY B-41, NIZAMUDDIN EAST, NEW DELHI - 110013.