Workshop Is Designed for Following Completed Registration Form Has to Be Forwarded GGCC LLPP Workshop Professionals: to Workshop Co-Ordinator Along with Prescribed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vividh Bharati Was Started on October 3, 1957 and Since November 1, 1967, Commercials Were Aired on This Channel
22 Mass Communication THE Ministry of Information and Broadcasting, through the mass communication media consisting of radio, television, films, press and print publications, advertising and traditional modes of communication such as dance and drama, plays an effective role in helping people to have access to free flow of information. The Ministry is involved in catering to the entertainment needs of various age groups and focusing attention of the people on issues of national integrity, environmental protection, health care and family welfare, eradication of illiteracy and issues relating to women, children, minority and other disadvantaged sections of the society. The Ministry is divided into four wings i.e., the Information Wing, the Broadcasting Wing, the Films Wing and the Integrated Finance Wing. The Ministry functions through its 21 media units/ attached and subordinate offices, autonomous bodies and PSUs. The Information Wing handles policy matters of the print and press media and publicity requirements of the Government. This Wing also looks after the general administration of the Ministry. The Broadcasting Wing handles matters relating to the electronic media and the regulation of the content of private TV channels as well as the programme matters of All India Radio and Doordarshan and operation of cable television and community radio, etc. Electronic Media Monitoring Centre (EMMC), which is a subordinate office, functions under the administrative control of this Division. The Film Wing handles matters relating to the film sector. It is involved in the production and distribution of documentary films, development and promotional activities relating to the film industry including training, organization of film festivals, import and export regulations, etc. -

Laboratories Reporting to ICMR
भारतीय आयु셍वज्ञि ान अनुसंधान पररषद वा्य अनुसंधान 셍वभाग, वा्य और पररवार क쥍याण मंत्रालय, भारत सरकार Indian Council of Medical Research Department of Health Research, Ministry of Health and Family Welfare, Government of India Date: 28/07/2021 Total Operational (initiated independent testing) Laboratories reporting to ICMR: Government laboratories : 1297 Private laboratories : 1494 - Real-Time RT PCR for COVID-19 : 1705 (Govt: 623 + Private: 1082) - TrueNat Test for COVID-19 : 938 (Govt: 625 + Private: 313) - CBNAAT Test for COVID-19 : 130 (Govt: 41 + Private: 89) - Other Molecular-Nucleic Acid (M-NA) Testing Platforms for COVID-19 : 18 (Govt: 08 + Private: 10) Note: Other Molecular-Nucleic Acid includes Abbott ID NOW, RT-LAMP, CRISPR-Cas9 and Accula™ Total No. of Labs : 2791 *CSIR/DBT/DST/DAE/ICAR/DRDO/MHRD/ISRO Laboratories. #Laboratories approved for both Real-Time RT-PCR and TrueNat/CBNAAT $Laboratories approved for both TrueNAT and CBNAAT ¥ Laboratories approved for Abbott ID NOW alone or in combination with any other testing platforms @Laboratories approved for RT-LAMP alone or in combination with any other testing platforms € Laboratories approved for CRISPR-Cas9 alone or in combination with any other testing platforms δ Laboratories approved for Accula™ alone or in combination with any other testing platforms P: Provisional Δ Pvt. Laboratories acquired by Govt. 1 | P a g e S. Test Names of States Names of Government Institutes Names of Private Institutes No. Category 1. Andhra Pradesh RT-PCR 1. Sri Venkateswara Institute of Medical 1. Manipal Hospital, Tadepalli, Guntur (131) Sciences, Tirupati 2. -

Current Affairs Magazine We Are Trying Our Best to Be a ADVISORS Facilitator Cum Mentor in Your Journey to Be Knowledgeable
MONTHLY ISSUE - OCTOBER - 2015 CurrVanik’s ent Affairs Banking | Railway | Insurance | SSC | UPSC | OPSC | PSU UAE Visit New OPSC OCS-2015 Special Volume-2y Govt. of India aunched b New Scheme L Two Practice Set for IBPS-PO (Preliminary) One Practice Set for IBPS-PO (Main) 40 MCQs on Computers Vanik’s Page 200 Updated MCQs 100 One Liners 100 GK for SSC & Railway Vanik’s Knowledge Garden Leading Institute for Banking, Railway & SSC P u b l i c a t i o n s VANIK'S PAGE COUNTRY, CAPITAL & CURRENCY European Coun tries Capital Currency North Capital Currency United Kingdom London Pound Sterling Americ an Nations France Paris Euro Antigua and Barbuda St. John's East Caribbean Spain Madrid Euro dollar Portugal Lisbon Euro The Bahamas Nassau Bahamian dollar Germany Berlin Euro Barbados Bridgetown Barbadian dollar Italy Rome Euro Belize Belmopan Belize dollar Vatican City Vatican Euro Canada Ottawa Canadian dollar Malta Valletta Euro Costa Rica San José Costa Rican colón Switzerland Bern Swiss Franc Cuba Havana Peso Belgium Brussels Euro Dominica Roseau East Caribbean Netherlands Amsterdam Euro dollar Denmark Copenhagen Krone Dominican Republic Santo Dominican Peso Norway Oslo Norwegian krone Domingo Sweden Stockholm Krona El Salvador San Salvador United States dollar Finland Helsinki Euro Grenada St. George's East Caribbean Estonia Tallinn Euro dollar Latvia Riga Euro Guatemala Guatemala Guatemalan quetzal Lithuania Vilnius Euro City Belarus Minsk Belarusian ruble Haiti Port-au-Prince Haitian gourde Ukraine Kiev Ukrainian hryvnia Poland Warsaw -
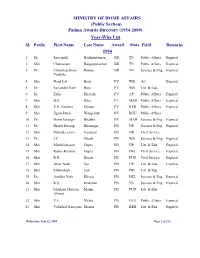
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

Eminent HIV Researcher Dr Suniti Solomon Passes Away at 76
Eminent HIV researcher Dr Suniti Solomon passes away at 76 29 July 2015 | News | By Srinivas Rasoor Eminent HIV researcher Dr Suniti Solomon passes away at 76 Image not found or type unknown The medical fraternity, scientists and biologists are mourning over the sad demise of India's most respectable HIV researcher and the President of the AIDS Society of India, Dr. Suniti Solomon. Dr. Solomon, who was ailing from liver cancer, passed away at her residence in Chennai on Tuesday. She was 76 years old and was the first to bring to the world the prevalence of HIV infection in India in 1986. She was also the first one to successfully lead the research into the treatment of HIV infected individuals. "Her death was sudden, she passed away this morning. She was getting treatment for liver cancer for the past two months," Ganesh and Krishnan of Y R Gaitonde Center for AIDS Research and Education (YRG Care) told media. Dr. Suniti Solomon earned an M.D in Microbiology from Madras University. She was trained in pathology in the United Kingdom and the United States. She was the Founder-Director of the Y.R. Gaitonde Center for AIDS Research and Education (YRG CARE), a premier HIV/AIDS care and support centre in Chennai. She and her colleagues documented the first evidence of the HIV infection in India in 1986. When she served the Madras Medical College and Government General Hospital as a Professor of Microbiology, she set up the first voluntary testing and counseling centre and an AIDS Research Group in Chennai. -

Cme Invitation Design
AUTOIMMUNE DISEASES Diagnosis and Interpretation Organized By Date March 23, 2014 Venue Microbiological Laboratory IMA Hall, (NABL Accredited), Coimbatore Syrian Church Road, & Coimbatore Indian Association of Medical Microbiologists Time Tamil Nadu Pondicherry Chapter 9am - 6pm Mariappa Mani Chairman-CME, Managing Director-Microbiological Laboratory .................................................................................................................................................. In an autoimmune reaction, the immune system attacks thyself by mistake and harms the body's own tissues. The autoimmune diseases comprise a group of immunologic disorders whose common denominator is the presence of an idiopathic systemic autoimmune process. The MICROBIOLOGICAL LABORATORY, Coimbatore provides a comprehensive panel of diagnostic services using the latest scientific discoveries to provide innovative methods in the detection, diagnosis and treatment assessment of immunologic, hematologic, pathologic, biochemical and oncologic disease states. A quiet revolution in the field of autoimmunity is underway. Its objective is to provide simple and accurate results for making laboratory diagnosis of autoimmune diseases. Although the autoimmune diseases share many common features and clinical presentations, differentiation between the diseases is crucial because of important distinction in clinical course, appropriate treatment and prognosis. Hence, there is urgent need to review and update the current scenario on clinical manifestation, laboratory -

List of Fellows (Name-Wise) Upto 2016
LIST OF FELLOWS (NAME-WISE) UPTO 2016 0. Description Year 1. Abdul Kalam, A.P.J. Biomedical Engineering July 1995 DMIT. Former President, Republic of India. Res: 10 Rajaji Marg, New Delhi-110001. Permanent Address: No. 2, Mosque Street, Rameswaram, Ramanathapuram District, Tamil Nadu-623526. Tel: Off: (011) 3015321, 3014930, Res: (04567) 6493708, Fax: 2300756, E-mail: [email protected] (b 1931) (d.2015) Gen. Amir Chand Oration (NAMS, 1997-98) Padma Bhushan (1981); Padma Vibhushan (1990); Bharat Ratna (1997); D.Sc (h.c.) from several Universities; National Design Award; Dr. Biren Roy Space Award; Om Prakash Bhasin Award; National Nehru Award by Govt. of Madhya Pradesh; GM Modi Award for Science 1996; HK Firodia Award for Excellence in S&T 1996; Veer Savarkar Award 1998; Hon Fellow-Institution of Electronics and Telecommunication Engineers. 2. Abraham, Jacob Neurosurgery 1984 MS, MS (Neuro), FACS, FACA. Res: 10, 15th Avenue, Harrington Road, Chennai- 600031. Tel: Res: (044) 28363211, 42849258, Mobile: 09940118382, E-mail: [email protected] (b.1931). Basanti Devi Amir Chand Prize (ICMR, 1984); Sachs Memorial Lecturer, USA (1989). 3. Achari, Kamala Obstetrics and Gynecology 1982 MS, FRCOG, FICS, FACS. Emeritus Professor, Patna Medical College, Patna-800001 (Bihar). Res: 'Tirumalai', 21/D Road No.10, Rajendra Nagar, Patna- 800016. (b.1924) (d. 2014). 4. Adithan, C. Pharmacology July 2003 MD, PhD, FIMSA, FIPS. Former Professor & Head, Department of Pharmacology, Jawaharlal Institute of Postgraduate Medical Education & Research, Pondicherry- 605006. Currently: Director-CIDRF and Professor of Pharmacology, Mahatma Gandhi Medical College and Research Institute, Pondicherry-607403. Res: Flat No. 1, Srinivas Towers, Vazhudavour Road, Kathirkamam, Pondicherry-605009. -

Dr. Balachandar Kathirvelu,M.B.B.S, Ph.D
Dr. Balachandar Kathirvelu, M.B.B.S, Ph.D. Clinical Assistant Professor, Rehabilitation Sciences Office: 915-747-7260 University of Texas at El Paso Email: [email protected] Office: 1101 N Campbell St. Room 306 [email protected] El Paso, Texas 79902 EDUCATION Ph.D., Tulane University, New Orleans, LA, USA Behavioral Neuroscience, 2013 Advisor: Dr. Paul J. Colombo Dissertation: Investigations of the role of the transcription factor CREB in memory formation, and interactions between the hippocampus and the striatum memory systems M.S., Tulane University, New Orleans, LA, USA Behavioral Neuroscience, 2010 Thesis: Learning-induced changes in CREB phosphorylation during memory formation: evidence for interaction between the hippocampus and dorsal striatum M.B.B.S. The Tamil Nadu Dr. M.G.R. Medical University Bachelor of Medicine, Bachelor of Surgery, 2002 Kilpauk Medical College, Chennai, TN, INDIA PROFESSIONAL APPOINTMENTS Clinical Asst Prof. University of Texas at El Paso 06/ 2019 - Present Rehabilitation Sciences Instructor of Central Michigan University, College of Medicine Anatomy 08/2018 -05/2019 Postdoctoral Central Michigan University, Neuroscience Program Research Associate 11/2017 – 05/2019 Postdoctoral Scholar The University of California, Los Angeles (UCLA) 2012 – 2017 David Geffen School of Medicine, Department of Neurology Page 1 of 6 Teaching Assistant Tulane University 2006 –2012 Neuroscience program & Department of Psychology Research Assistant Department of Public Health and Preventive medicine, Chennai, 2004 – 2005 -

Medical Research Foundation Notice
MEDICAL RESEARCH FOUNDATION To The Members of the Medical Research Foundation 7th September 2011 NOTICE NOTICE IS HEREBY GIVEN TO THE MEMBERS OF THE MEDICAL RESEARCH FOUNDATION THAT THE 33RD ANNUAL GENERAL MEETING OF THE FOUNDATION WILL BE HELD ON THRUSDAY, THE 29th SEPTEMBER 2011 AT 6.00 P.M. AT BOARD ROOM, 2ND FLOOR, KAMALNAYAN BAJAJ RESEARCH CENTRE BLOCK, NEW NO 41, OLD NO 18, COLLEGE ROAD, CHENNAI 600 006. THE AGENDA FOR THE MEETING IS GIVEN BELOW: AGENDA 1. To consider and adopt the Annual report of the Foundation for the year 2010-11. 2. To consider and adopt the Audited Income and Expenditure Account for the year ended March 31, 2011 and the Balance Sheet as at that date together with the Report of the Auditors thereon. 3. To appoint Auditors and to fix their remuneration for the year ending 31st March 2012. 4. To elect Members to the Board of Management in the vacancies caused by the retirement of the following Members, who are eligible for re-appointment. Name Category Mr. H D Malesra Patron Member Mr.Mani S Subramonian Patron Member 5. To elect Members to the Board of Management in the existing vacancy. Kindly make it convenient to attend the meeting. N.SUGALCHAND JAIN HONY. SECRETARY & TREASURER 1 MEDICAL RESEARCH FOUNDATION SANKARA NETHRALAYA MISSION STATEMENT QUALITY OBJECTIVES The mission of Sankara Nethralaya is to To maintain the quality of ophthalmic provide Total Eye-care solutions of highest s e r v i c e s i n a c c o r d a n c e w i t h standards to all sections of community international standards. -

International Partners of ICMR an Overview of International
International Partners of ICMR An Overview of International Collaborative Projects in Health Research approved by Health Ministry’s Screening Committee (HMSC) during August, 2017 to July, 2020 (Volume V) International Health Division INDIAN COUNCIL OF MEDICAL RESEARCH, NEW DELHI Conceptualization, Design & Layout Mukesh Kumar, Harpreet Sandhu, Reema Roshan, Pratima Verma, Imran Ahmad, Rishi Chaudhary August, 2020 International Health Division (IHD), ICMR, Ansari Nagar, New Delhi-110029 Email: [email protected] Dated the 14th August, 2020 Foreword I am pleased to know that the International Health Division (IHD) of ICMR is publishing the fifth volume of the document entitled “An Overview of International Collaborative Research Projects in Health Research approved by Health Ministry’s Screening Committee (HMSC) during August, 2017 to July, 2020”. This document is in continuation of previous four volumes published by International Health Division in 2007, 2013, 2015, 2017, which have provided information on the international collaborative research projects approved by HMSC from January 2000 to July 2017. The earlier volumes released by IHD, enlisted the collaborative research projects undertaken by Scientists & Researchers in India in collaboration with their foreign partner scientists with foreign assistance and/or collaboration. The current document provides information about projects approved by HMSC during August 2017 to July 2020 alongwith information on ICMR’s international partnerships with different countries/agencies and details on the areas of interest and modes of collaboration. The recent decision taken for the organization of HMSC meetings at every alternate month and complete shifting over to the online submission/processing/review and consideration of international collaborative projects by the Health Ministry’s Screening Committee have further streamlined the procedures by cutting down the delays. -

NAME: Dr. D. FEBE RENJITHA SUMAN DEGREE:MD (Pathology)
NAME: Dr. D. FEBE RENJITHA SUMAN DEGREE:M.D. (Pathology) & Fellowship in HIV Medicine (FHM) DESIGNATION: Professor of Pathology AREA OF EXPERTISE: Haematology & Clinical Pathology PROJECTS CONDUCTED : - Clinico haematological study of thrombophilic markers-2019 - Finding an early marker for diagnosis of sepsis, 2019 - Rapid and Reliable?-An analysis of point of care prothrombin time testing,2019 - Clot wave form analysis in dengue –Presented a poster at National malaria and dengue conference,Kuala Lumpur,Malaysia,2017 - Clot wave form analysis in sepsis- BinojC, Final year MBBS was awarded summer research fellowship 2017 - Screening for haemoglobinopathies in anemic pregnant women of -2017 - Spectrum of protein C deficiency in a tertiary care hospital,2017 - Can clinical coagulometer help in drug monitoring,2017 - Placental pathology and neutrophil traps in antiphospholipid syndrome-A comparative studies with antiphospholipid like conditions-2018 - Hematological variations among twins -2018 - Developing a model screening program for antenatal women to prevent thalassemia – 2018 - Clot wave form analysis in dengue 2017 - Clot wave form analysis in sepsis- 2017 - Screening for haemoglobinopathies in anemic pregnant women of -2017 - Spectrum of protein C deficiency in a tertiary care hospital,2017 - Can clinical coagulometer help in drug monitoring,2017 - Utility of Cell population data in dengue –2016 - Profile of paediatric chronic myeloid leukemia - Prognostic significance of RHAMM ( CD168) expression in pediatric acute leukemias - - Correlation -

COVID-19 Testing Labs
भारतीय आयु셍वज्ञि ान अनुसधं ान पररषद वा्य अनुसंधान 셍वभाग, वा्य और पररवार क쥍याण मंत्रालय, भारत सरकार Date: 20/05/2020 Total Operational (initiated independent testing) Laboratories reporting to ICMR: Government laboratories : 396 Private laboratories : 173 - Real-Time RT PCR for COVID-19 : 437 (Govt: 294 + Private: 143) - TrueNat Test for COVID-19 : 81 (Govt: 76 + Private: 05) - CBNAAT Test for COVID-19 : 51 (Govt: 26 + Private: 25) Total No. of Labs : 569 *CSIR/DBT/DST/DAE/ICAR/DRDO Laboratories. #Laboratories approved for both Real-Time RT-PCR and TrueNat/CBNAAT $Laboratories approved for both TrueNAT and CBNAAT S. Names of Test Names of Government Institutes Names of Private Institutes No. States Category 1. Andhra RT-PCR 1. Sri Venkateswara Institute of Medical Pradesh (52) Sciences, Tirupati 2. Sri Venkateswara Medical College, Tirupati 1 | P a g e भारतीय आयु셍वज्ञि ान अनुसधं ान पररषद वा्य अनुसंधान 셍वभाग, वा्य और पररवार क쥍याण मंत्रालय, भारत सरकार S. Names of Test Names of Government Institutes Names of Private Institutes No. States Category 3. Rangaraya Medical College, Kakinada 4. #Sidhartha Medical College, Vijaywada 5. Govt. Medical College, Ananthpur 6. Guntur Medical College, Guntur 7. Rajiv Gandhi Institute of Medical Sciences, Kadapa 8. Andhra Medical College, Visakhapatnam 9. Govt. Kurnool Medical College, Kurnool 10. Govt. Medical College, Srikakulam TrueNat 11. Damien TB Research Centre, Nellore 12. SVRR Govt. General Hospital, Tirupati 13. Community Health Centre, Gadi Veedhi Saluru, Vizianagaram 14. Community Health Centre, Bhimavaram, West Godavari District 15.