Volume II) January, 2008 to December, 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vividh Bharati Was Started on October 3, 1957 and Since November 1, 1967, Commercials Were Aired on This Channel
22 Mass Communication THE Ministry of Information and Broadcasting, through the mass communication media consisting of radio, television, films, press and print publications, advertising and traditional modes of communication such as dance and drama, plays an effective role in helping people to have access to free flow of information. The Ministry is involved in catering to the entertainment needs of various age groups and focusing attention of the people on issues of national integrity, environmental protection, health care and family welfare, eradication of illiteracy and issues relating to women, children, minority and other disadvantaged sections of the society. The Ministry is divided into four wings i.e., the Information Wing, the Broadcasting Wing, the Films Wing and the Integrated Finance Wing. The Ministry functions through its 21 media units/ attached and subordinate offices, autonomous bodies and PSUs. The Information Wing handles policy matters of the print and press media and publicity requirements of the Government. This Wing also looks after the general administration of the Ministry. The Broadcasting Wing handles matters relating to the electronic media and the regulation of the content of private TV channels as well as the programme matters of All India Radio and Doordarshan and operation of cable television and community radio, etc. Electronic Media Monitoring Centre (EMMC), which is a subordinate office, functions under the administrative control of this Division. The Film Wing handles matters relating to the film sector. It is involved in the production and distribution of documentary films, development and promotional activities relating to the film industry including training, organization of film festivals, import and export regulations, etc. -

Workshop Is Designed for Following Completed Registration Form Has to Be Forwarded GGCC LLPP Workshop Professionals: to Workshop Co-Ordinator Along with Prescribed
WHO SHOULD PARTICIPATE? REGISTRATION This workshop is designed for following Completed Registration form has to be forwarded GGCC LLPP Workshop professionals: to Workshop Co-ordinator along with prescribed 18-20 June 2010 Microbiologists, Pathologists and Biochemists. Fee of Rs.3000 in the form of DD. DD should be Lab Directors / Managers and Laboratory drawn in favor of “YRG CARE” payable at Chennai. Technologists. The fee includes registration, workshop materials, QA/QC personnel, Quality Officers and Quality refreshments, breakfast and lunch provided during OVERVIEW Managers. the workshop. GCLP outline the principles and procedures to be Professionals associated with clinical The seats are restricted to 60 and participants will followed by medical laboratories involved in patient laboratory management and accreditation. be registered on “first come-first served basis” . care and/or clinical research so as to provide Only those who are currently engaged in the The registration DOES NOT cover accommodation, consistent, reproducible, auditable, and reliable diagnostic lab or clinical research are encouraged however, assistance will be provided for laboratory results; which contribute to good patient to participate in this workshop and students are arrangement of accommodation on request. not eligible. care and promote a positive attitude toward testing from a patient’s perspective. This workshop is WORKSHOP CONTENTS VENUE designed to offer comprehensive guidance for The key contents of the workshop are: those who are implementing GCLP in their TICEL Bio Park Ltd Principles of quality essentials Taramani Road, Taramani laboratories. QA/QC practices Chennai - 600113 Establishment and management of quality LEARNING OBJECTIVES ORGANIZING COMMITTEE system Documentation structure and system Organizing Chair Learn GCLP principles and their relation to Test facility operation Prof. -

Current Affairs Magazine We Are Trying Our Best to Be a ADVISORS Facilitator Cum Mentor in Your Journey to Be Knowledgeable
MONTHLY ISSUE - OCTOBER - 2015 CurrVanik’s ent Affairs Banking | Railway | Insurance | SSC | UPSC | OPSC | PSU UAE Visit New OPSC OCS-2015 Special Volume-2y Govt. of India aunched b New Scheme L Two Practice Set for IBPS-PO (Preliminary) One Practice Set for IBPS-PO (Main) 40 MCQs on Computers Vanik’s Page 200 Updated MCQs 100 One Liners 100 GK for SSC & Railway Vanik’s Knowledge Garden Leading Institute for Banking, Railway & SSC P u b l i c a t i o n s VANIK'S PAGE COUNTRY, CAPITAL & CURRENCY European Coun tries Capital Currency North Capital Currency United Kingdom London Pound Sterling Americ an Nations France Paris Euro Antigua and Barbuda St. John's East Caribbean Spain Madrid Euro dollar Portugal Lisbon Euro The Bahamas Nassau Bahamian dollar Germany Berlin Euro Barbados Bridgetown Barbadian dollar Italy Rome Euro Belize Belmopan Belize dollar Vatican City Vatican Euro Canada Ottawa Canadian dollar Malta Valletta Euro Costa Rica San José Costa Rican colón Switzerland Bern Swiss Franc Cuba Havana Peso Belgium Brussels Euro Dominica Roseau East Caribbean Netherlands Amsterdam Euro dollar Denmark Copenhagen Krone Dominican Republic Santo Dominican Peso Norway Oslo Norwegian krone Domingo Sweden Stockholm Krona El Salvador San Salvador United States dollar Finland Helsinki Euro Grenada St. George's East Caribbean Estonia Tallinn Euro dollar Latvia Riga Euro Guatemala Guatemala Guatemalan quetzal Lithuania Vilnius Euro City Belarus Minsk Belarusian ruble Haiti Port-au-Prince Haitian gourde Ukraine Kiev Ukrainian hryvnia Poland Warsaw -
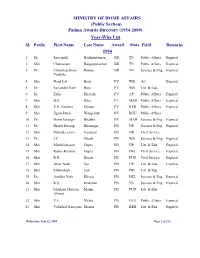
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

Non-Traditional Lifestyles and Prevalence of Mental Disorders in Adolescents in Goa, India Aravind Pillai, Vikram Patel, Percy Cardozo, Robert Goodman, Helen A
The British Journal of Psychiatry (2008) 192, 45–51. doi: 10.1192/bjp.bp.106.034223 Non-traditional lifestyles and prevalence of mental disorders in adolescents in Goa, India Aravind Pillai, Vikram Patel, Percy Cardozo, Robert Goodman, Helen A. Weiss and Gracy Andrew Background Adolescents comprise a fifth of the population of India, but disorders (1.0%), depressive disorder (0.5%), behavioural there is little research on their mental health. We conducted disorder (0.4%) and attention-deficit hyperactivity disorder an epidemiological study in the state of Goa to describe the (0.2%). Adolescents from urban areas and girls who faced current prevalence of mental disorders and its correlates gender discrimination had higher prevalence. The final among adolescents aged between 12 and 16 years. multivariate model found an independent association of mental disorders with an outgoing ‘non-traditional’ lifestyle Aims (frequent partying, going to the cinema, shopping for fun and To estimate the prevalence and correlates of mental having a boyfriend or girlfriend), difficulties with studies, lack disorders in adolescents. of safety in the neighbourhood, a history of physical or verbal abuse and tobacco use. Having one’s family as the Method Population-based survey of all eligible adolescents from six primary source of social support was associated with lower urban wards and four rural communities which were prevalence of mental disorders. randomly selected. We used a Konkani translation of the Development and Well-Being Assessment to diagnose Conclusions current DSM–IV emotional and behavioural disorders. All The current prevalence of mental disorders in adolescents in adolescents were also interviewed on socio-economic our study was very low compared with studies in other factors, education, neighbourhood, parental relations, peer countries. -

Speaker Spotlight: Vikram Patel, Harvard Medical School
Speaker Spotlight: Vikram Patel, Harvard Medical School Dr Vikram Patel is the Co-Founder and former Director for the Centre for Global Mental Health at the London School of Hygiene and Tropical Medicine (LSHTM). He is also Co-Director of the Centre for Control of Chronic Conditions at the Public Health Foundation of India and the Co- Founder of Sangath, an Indian NGO dedicated to research in the areas of child development, adolescent health, and mental health. Listed as one of the world's 100 most influential people by TIME magazine, Dr. Patel’s work spans a wide variety of topics and disciplines, however his primary interest is in global mental health, specifically the improved treatment and care of people with mental disorders around the globe. He will deliver a keynote presentation in this vein at the Global Mental Health Conference: Tuesday 6th June 2017 1.45pm – 2.30pm Dr Vikram Patel (Harvard Medical School) Psychological treatments for the world: What rich countries can learn from the global south More about Dr Patel Dr Patel currently serves on three World Health Organisation Committees including Mental Health as well as on four Government of India Committees, including the Mental Health Policy Group (which drafted India’s first national mental health policy, launched on October 10th, 2014). He is Co-founder and Member of the Managing Committee for Sangath, a mental health research NGO located in Goa that works with the LSHTM on multiple projects focusing on child development, adolescent health, and mental health. In 2008, Sangath won the MacArthur Foundation’s International Prize for Creative and Effective Institutions and is now using the grant money to pioneer various ways in which task-sharing in mental health care can be properly distributed between primary care professionals and community based workers. -

Primary Care BMJ: First Published As 10.1136/Bmj.38442.636181.E0 on 3 May 2005
Primary care BMJ: first published as 10.1136/bmj.38442.636181.E0 on 3 May 2005. Downloaded from Chronic fatigue in developing countries: population based survey of women in India Vikram Patel, Betty Kirkwood, Helen Weiss, Sulochana Pednekar, Janice Fernandes, Bernadette Pereira, Medha Upadhye, David Mabey London School of Abstract and nutritional supplements to treat the symptom pre- Hygiene and Tropical Medicine, sumptively. Such preparations account for the largest Objectives To describe the prevalence of and risk 2 London category of drugs dispensed in South Asia. WC1E 7HT factors for chronic fatigue in a developing country; in Little research has been done on the associations of Vikram Patel particular, to determine the association of anaemia, fatigue with psychological factors in developing coun- reader in mental health, and gender disadvantage factors with international mental tries, particularly in the context of the high prevalence health chronic fatigue. of anaemia and poor nutrition. We hypothesised that Betty R Kirkwood Design Community survey. the principal association of fatigue was with psycho- professor of Setting Primary health centre catchment area in Goa, epidemiology and social risk factors, similar to patterns seen in developed India. 3 international health countries, and with factors reflecting gender disadvan- Helen Weiss Participants 3000 randomly sampled women aged 18 tage that are important determinants of women’s senior lecturer in to 50 years. health.45 epidemiology and Main outcome measures Data on the primary statistics David Mabey outcome (reporting of fatigue for at least six months) professor of and psychosocial exposures elicited by structured Methods communicable interview; presence of anaemia determined from a diseases Participants blood sample. -

Eminent HIV Researcher Dr Suniti Solomon Passes Away at 76
Eminent HIV researcher Dr Suniti Solomon passes away at 76 29 July 2015 | News | By Srinivas Rasoor Eminent HIV researcher Dr Suniti Solomon passes away at 76 Image not found or type unknown The medical fraternity, scientists and biologists are mourning over the sad demise of India's most respectable HIV researcher and the President of the AIDS Society of India, Dr. Suniti Solomon. Dr. Solomon, who was ailing from liver cancer, passed away at her residence in Chennai on Tuesday. She was 76 years old and was the first to bring to the world the prevalence of HIV infection in India in 1986. She was also the first one to successfully lead the research into the treatment of HIV infected individuals. "Her death was sudden, she passed away this morning. She was getting treatment for liver cancer for the past two months," Ganesh and Krishnan of Y R Gaitonde Center for AIDS Research and Education (YRG Care) told media. Dr. Suniti Solomon earned an M.D in Microbiology from Madras University. She was trained in pathology in the United Kingdom and the United States. She was the Founder-Director of the Y.R. Gaitonde Center for AIDS Research and Education (YRG CARE), a premier HIV/AIDS care and support centre in Chennai. She and her colleagues documented the first evidence of the HIV infection in India in 1986. When she served the Madras Medical College and Government General Hospital as a Professor of Microbiology, she set up the first voluntary testing and counseling centre and an AIDS Research Group in Chennai. -

The Urban Morphology of Hyderabad, India: a Historical Geographic Analysis
Western Michigan University ScholarWorks at WMU Master's Theses Graduate College 6-2020 The Urban Morphology of Hyderabad, India: A Historical Geographic Analysis Kevin B. Haynes Western Michigan University, [email protected] Follow this and additional works at: https://scholarworks.wmich.edu/masters_theses Part of the Human Geography Commons, and the Remote Sensing Commons Recommended Citation Haynes, Kevin B., "The Urban Morphology of Hyderabad, India: A Historical Geographic Analysis" (2020). Master's Theses. 5155. https://scholarworks.wmich.edu/masters_theses/5155 This Masters Thesis-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Master's Theses by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THE URBAN MORPHOLOGY OF HYDERABAD, INDIA: A HISTORICAL GEOGRAPHIC ANALYSIS by Kevin B. Haynes A thesis submitted to the Graduate College in partial fulfillment of the requirements for the degree of Master of Science Geography Western Michigan University June 2020 Thesis Committee: Adam J. Mathews, Ph.D., Chair Charles Emerson, Ph.D. Gregory Veeck, Ph.D. Nathan Tabor, Ph.D. Copyright by Kevin B. Haynes 2020 THE URBAN MORPHOLOGY OF HYDERABAD, INDIA: A HISTORICAL GEOGRAPHIC ANALYSIS Kevin B. Haynes, M.S. Western Michigan University, 2020 Hyderabad, India has undergone tremendous change over the last three centuries. The study seeks to understand how and why Hyderabad transitioned from a north-south urban morphological directional pattern to east-west during from 1687 to 2019. Satellite-based remote sensing will be used to measure the extent and land classifications of the city throughout the twentieth and twenty-first century using a geographic information science and historical- geographic approach. -

List of Fellows (Name-Wise) Upto 2016
LIST OF FELLOWS (NAME-WISE) UPTO 2016 0. Description Year 1. Abdul Kalam, A.P.J. Biomedical Engineering July 1995 DMIT. Former President, Republic of India. Res: 10 Rajaji Marg, New Delhi-110001. Permanent Address: No. 2, Mosque Street, Rameswaram, Ramanathapuram District, Tamil Nadu-623526. Tel: Off: (011) 3015321, 3014930, Res: (04567) 6493708, Fax: 2300756, E-mail: [email protected] (b 1931) (d.2015) Gen. Amir Chand Oration (NAMS, 1997-98) Padma Bhushan (1981); Padma Vibhushan (1990); Bharat Ratna (1997); D.Sc (h.c.) from several Universities; National Design Award; Dr. Biren Roy Space Award; Om Prakash Bhasin Award; National Nehru Award by Govt. of Madhya Pradesh; GM Modi Award for Science 1996; HK Firodia Award for Excellence in S&T 1996; Veer Savarkar Award 1998; Hon Fellow-Institution of Electronics and Telecommunication Engineers. 2. Abraham, Jacob Neurosurgery 1984 MS, MS (Neuro), FACS, FACA. Res: 10, 15th Avenue, Harrington Road, Chennai- 600031. Tel: Res: (044) 28363211, 42849258, Mobile: 09940118382, E-mail: [email protected] (b.1931). Basanti Devi Amir Chand Prize (ICMR, 1984); Sachs Memorial Lecturer, USA (1989). 3. Achari, Kamala Obstetrics and Gynecology 1982 MS, FRCOG, FICS, FACS. Emeritus Professor, Patna Medical College, Patna-800001 (Bihar). Res: 'Tirumalai', 21/D Road No.10, Rajendra Nagar, Patna- 800016. (b.1924) (d. 2014). 4. Adithan, C. Pharmacology July 2003 MD, PhD, FIMSA, FIPS. Former Professor & Head, Department of Pharmacology, Jawaharlal Institute of Postgraduate Medical Education & Research, Pondicherry- 605006. Currently: Director-CIDRF and Professor of Pharmacology, Mahatma Gandhi Medical College and Research Institute, Pondicherry-607403. Res: Flat No. 1, Srinivas Towers, Vazhudavour Road, Kathirkamam, Pondicherry-605009. -

Annual Report 2016 - 17
FORUM FOR MEDICAL ETHICS SOCIETY Trust registration No. F-17441 (Mumbai), 1995 Society registration No. Mumbai, 218, 1995, GBSD Annual Report 2016 - 17 www.ijme.in Registered address: O-18, Nav Bhavna Premises CHSL, SVS Marg, Prabhadevi, Mumbai - 400 025. Contact address: C/o Shantilal Patel, C-3, 3rd Floor, Nav Bhavna CHSL, SVS Marg, Prabhadevi, Mumbai - 400 025. Tel.: 07506265856 E: [email protected]; [email protected]; [email protected] MANAGING COMMITTEE, FMES The present Managing Committee was elected at the Annual General Meeting, July 16, 2016. The members elected were: Sanjay Nagral (Chairperson) Sunita VS Bandewar (Secretary) Rakhi Ghoshal (Jt Secretary) Lubna Duggal (Treasurer) Leni Chaudhuri (Member) Barun Mukhopadhyay (Member) Shamim Modi (Member) Shyamala Nataraj (Member) Sunita Simon Kurpad (Member) EDITORIAL BOARD, IJME Editor Emeritus Sunil Pandya Editor Amar Jesani Consulting Editor Sandhya Srinivasan Working Editors Mala Ramanathan Rakhi Ghoshal Sanjay A Pai Sunita VS Bandewar Vijayaprasad Gopichandran Editorial Board Aamir Jafarey, Pakistan Jacqueline Chin Joon Lin, Singapore Sabina Faiz Rashid, Bangladesh Aasim Ahmad, Pakistan Jing Bao Nie, New Zealand Sanjay Nagral, Mumbai Abha Saxena, Switzerland Joy Akoijam, Imphal Silke Schicktanz, Germany Alex John London, USA Julian Sheather, UK Sisira Siribaddana, Sri Lanka Alok Sarin, Delhi Mario Vaz, Bengaluru Sreekumar N, Chennai Amit Sengupta, Delhi Neha Madhiwalla, Mumbai Sridevi Seetharam, Mysuru Angus Dawson, Australia Nithya Gogtay, Mumbai -

Srno Party Credit Grantor State Credit Grantor Branch Registered Address Outstanding Amount in Lacs Asset Classification Date Of
OUTSTANDING SRNO PARTY CREDIT GRANTOR STATE CREDIT GRANTOR BRANCH REGISTERED ADDRESS ASSET CLASSIFICATION DATE OF CLASSIFICATION OTHER BANK DIRECTOR 1 DIN FOR DIRECTOR 1 DIRECTOR 2 DIN FOR DIRECTOR 2 DIRECTOR 3 DIN FOR DIRECTOR 3 DIRECTOR 4 DIN FOR DIRECTOR 4 DIRECTOR 5 DIN FOR DIRECTOR 5 DIRECTOR 6 DIN FOR DIRECTOR 6 DIRECTOR 7 DIN FOR DIRECTOR 7 DIRECTOR 8 DIN FOR DIRECTOR 8 DIRECTOR 9 DIN FOR DIRECTOR 9 DIRECTOR 10 DIN FOR DIRECTOR 10 DIRECTOR 11 DIN FOR DIRECTOR 11 DIRECTOR 12 DIN FOR DIRECTOR 12 DIRECTOR 13 DIN FOR DIRECTOR 13 DIRECTOR 14 DIN FOR DIRECTOR 14 AMOUNT IN LACS 1 A & J IMPEX ALLAHABAD BANK PUNJAB ARMB CHANDIGARH E 124, phase -IV Focal point Ludhiana - 141002 1394 _ ANJU JAIN Survey No. 117, Kalgidhar Textile Mill Compound,Nr Kashiram Mill, Hotel Good Luck lane, Narol, 2 A A FABTEX PVT.LTD ALLAHABAD BANK GUJRAT AHMEDABAD SARDAR PATEL NAGAR Ahmadabad. – 382405 1560 Bank of Maharashtra Annmol B. Aggarwala 01259880 Shrikant B. Aggarwala 02204753 Gujarat E-3, MANGOLPURI INDUSTRIAL AREA, PHASE-II, DELHI- LEAD BANK: BANK OF JAYANT MOHANLAL 3 ALLIED PERFUMERS PVT LTD. ALLAHABAD BANK DELHI NEW DELHI INTERNATIONAL 4402 SANJAY JAIN 26147 RAJIV JAIN 26004 RAMESH SAREEN SANJEEV AGARWAL 25998 KAMAL KANT SHARMA ROHIT CHOWDHARY 26031 MOHAN GUPTA KAMAL KISHORE GUPTA 110034 BARODA, SBBJ,SBT GANDHI Reg. Office at 190, Poonamalle High Road, Chennai-600084 PNB, UBI, SBI, IDBI, KVB, Dr. B Arvind Shah PAN Dr. Chandra Ravindran V R MEHTA PAN SUDHIR CHANDRA PAN C M K REDDY PAN 4 Arvind Remedies Ltd ALLAHABAD BANK TAMIL NADU GEORGE TOWN CHENNAI 14,306.00 01063744 00771329 00051415 06602402 00859774 CIN NO: L24231TN1988PLC015882 IOB, CORPORATION AAVPS8498K PAN AAGPC2539N AFDPM6384D AEHPC1368N AFMPR3222G Rajkumar Singla PAN Saurav Singla PAN Anurag Singla PAN 5 BANKE BIHARI FLY ASH BRICKS ALLAHABAD BANK HARIYANA SOHNA, GURGAON Vill.