Clinical Trial Details (PDF Generation Date :- Tue, 22 Jun 2021 18
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Urban Morphology of Hyderabad, India: a Historical Geographic Analysis
Western Michigan University ScholarWorks at WMU Master's Theses Graduate College 6-2020 The Urban Morphology of Hyderabad, India: A Historical Geographic Analysis Kevin B. Haynes Western Michigan University, [email protected] Follow this and additional works at: https://scholarworks.wmich.edu/masters_theses Part of the Human Geography Commons, and the Remote Sensing Commons Recommended Citation Haynes, Kevin B., "The Urban Morphology of Hyderabad, India: A Historical Geographic Analysis" (2020). Master's Theses. 5155. https://scholarworks.wmich.edu/masters_theses/5155 This Masters Thesis-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Master's Theses by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THE URBAN MORPHOLOGY OF HYDERABAD, INDIA: A HISTORICAL GEOGRAPHIC ANALYSIS by Kevin B. Haynes A thesis submitted to the Graduate College in partial fulfillment of the requirements for the degree of Master of Science Geography Western Michigan University June 2020 Thesis Committee: Adam J. Mathews, Ph.D., Chair Charles Emerson, Ph.D. Gregory Veeck, Ph.D. Nathan Tabor, Ph.D. Copyright by Kevin B. Haynes 2020 THE URBAN MORPHOLOGY OF HYDERABAD, INDIA: A HISTORICAL GEOGRAPHIC ANALYSIS Kevin B. Haynes, M.S. Western Michigan University, 2020 Hyderabad, India has undergone tremendous change over the last three centuries. The study seeks to understand how and why Hyderabad transitioned from a north-south urban morphological directional pattern to east-west during from 1687 to 2019. Satellite-based remote sensing will be used to measure the extent and land classifications of the city throughout the twentieth and twenty-first century using a geographic information science and historical- geographic approach. -

Srno Party Credit Grantor State Credit Grantor Branch Registered Address Outstanding Amount in Lacs Asset Classification Date Of
OUTSTANDING SRNO PARTY CREDIT GRANTOR STATE CREDIT GRANTOR BRANCH REGISTERED ADDRESS ASSET CLASSIFICATION DATE OF CLASSIFICATION OTHER BANK DIRECTOR 1 DIN FOR DIRECTOR 1 DIRECTOR 2 DIN FOR DIRECTOR 2 DIRECTOR 3 DIN FOR DIRECTOR 3 DIRECTOR 4 DIN FOR DIRECTOR 4 DIRECTOR 5 DIN FOR DIRECTOR 5 DIRECTOR 6 DIN FOR DIRECTOR 6 DIRECTOR 7 DIN FOR DIRECTOR 7 DIRECTOR 8 DIN FOR DIRECTOR 8 DIRECTOR 9 DIN FOR DIRECTOR 9 DIRECTOR 10 DIN FOR DIRECTOR 10 DIRECTOR 11 DIN FOR DIRECTOR 11 DIRECTOR 12 DIN FOR DIRECTOR 12 DIRECTOR 13 DIN FOR DIRECTOR 13 DIRECTOR 14 DIN FOR DIRECTOR 14 AMOUNT IN LACS 1 A & J IMPEX ALLAHABAD BANK PUNJAB ARMB CHANDIGARH E 124, phase -IV Focal point Ludhiana - 141002 1394 _ ANJU JAIN Survey No. 117, Kalgidhar Textile Mill Compound,Nr Kashiram Mill, Hotel Good Luck lane, Narol, 2 A A FABTEX PVT.LTD ALLAHABAD BANK GUJRAT AHMEDABAD SARDAR PATEL NAGAR Ahmadabad. – 382405 1560 Bank of Maharashtra Annmol B. Aggarwala 01259880 Shrikant B. Aggarwala 02204753 Gujarat E-3, MANGOLPURI INDUSTRIAL AREA, PHASE-II, DELHI- LEAD BANK: BANK OF JAYANT MOHANLAL 3 ALLIED PERFUMERS PVT LTD. ALLAHABAD BANK DELHI NEW DELHI INTERNATIONAL 4402 SANJAY JAIN 26147 RAJIV JAIN 26004 RAMESH SAREEN SANJEEV AGARWAL 25998 KAMAL KANT SHARMA ROHIT CHOWDHARY 26031 MOHAN GUPTA KAMAL KISHORE GUPTA 110034 BARODA, SBBJ,SBT GANDHI Reg. Office at 190, Poonamalle High Road, Chennai-600084 PNB, UBI, SBI, IDBI, KVB, Dr. B Arvind Shah PAN Dr. Chandra Ravindran V R MEHTA PAN SUDHIR CHANDRA PAN C M K REDDY PAN 4 Arvind Remedies Ltd ALLAHABAD BANK TAMIL NADU GEORGE TOWN CHENNAI 14,306.00 01063744 00771329 00051415 06602402 00859774 CIN NO: L24231TN1988PLC015882 IOB, CORPORATION AAVPS8498K PAN AAGPC2539N AFDPM6384D AEHPC1368N AFMPR3222G Rajkumar Singla PAN Saurav Singla PAN Anurag Singla PAN 5 BANKE BIHARI FLY ASH BRICKS ALLAHABAD BANK HARIYANA SOHNA, GURGAON Vill. -
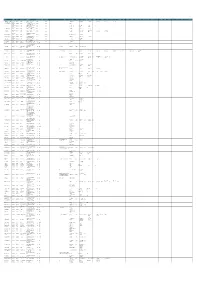
Srno Party Credit Grantor State Credit Grantor
OUTSTANDING SRNO PARTY CREDIT GRANTOR STATE CREDIT GRANTOR BRANCH REGISTERED ADDRESS ASSET CLASSIFICATION DATE OF CLASSIFICATION SUIT OTHER BANK DIRECTOR 1 DIN FOR DIRECTOR 1 DIRECTOR 2 DIN FOR DIRECTOR 2 DIRECTOR 3 DIN FOR DIRECTOR 3 DIRECTOR 4 DIN FOR DIRECTOR 4 DIRECTOR 5 DIN FOR DIRECTOR 5 DIRECTOR 6 DIN FOR DIRECTOR 6 DIRECTOR 7 DIN FOR DIRECTOR 7 DIRECTOR 8 DIN FOR DIRECTOR 8 DIRECTOR 9 DIN FOR DIRECTOR 9 DIRECTOR 10 DIN FOR DIRECTOR 10 DIRECTOR 11 DIN FOR DIRECTOR 11 DIRECTOR 12 DIN FOR DIRECTOR 12 DIRECTOR 13 DIN FOR DIRECTOR 13 DIRECTOR 14 DIN FOR DIRECTOR 14 DIN FOR DIRECTOR 14 AMOUNT IN LACS BIL INDUSTRIES LTD. EARLIER KNOWN AS ABHYUDAYA CO-OP. KHOPOLI PEN ROAD, VILLAGE- TAMBATTI, DIST. JANJID SHYAMKUMAR 1 MAHARASHTRA RECOVERY 1676.283692 12.04.1998 BAGLA SANTOSHKUMAR BAGLA SHIVKUMAR BAGLA LAXMINARAYAN K. S. PARK BHARAT BAGLA BHUPENDRA INDUSTRIES LTD BANK LTD. RAIGAD, STATE- MAHARASHTRA S. ABHYUDAYA CO-OP. 11/6, SHERE PUNJAB SOCIETY, MAHAKALI CAVES 2 GLOBAL TRADING CORPORATION MAHARASHTRA RECOVERY 378.32874 16.09.1996 SAWANT AMIT BANK LTD. ROAD, ANDHERI (E), MUMBAI- 400 093 ABHYUDAYA CO-OP. 401,GODAVARI BUILDING, SIR POCHKHANWALA MR. VISHRAM N. MR. BALU 3 J. SQUARE STEELS PVT. LTD MAHARASHTRA RECOVERY 6227.351795 15.12.2008 MR. RAJENDRA N. EKAMBE 675305 - 2320499 BANK LTD. ROAD, WORLI, MUMBAI 400 030 EKAMBE SURYAVANSHI ABHYUDAYA CO-OP. 343/A, BADAM WADI, 3RD FLOOR, ROOM NO.29, 4 LEON CONSULTANTS PVT. LTD MAHARASHTRA RECOVERY 195.20525 16.09.1996 DR. A. VELUMANI R. RAVINDRAN RITU G. HEGDE BANK LTD. -

Padma Vibhushan * * the Padma Vibhushan Is the Second-Highest Civilian Award of the Republic of India , Proceeded by Bharat Ratna and Followed by Padma Bhushan
TRY -- TRUE -- TRUST NUMBER ONE SITE FOR COMPETITIVE EXAM SELF LEARNING AT ANY TIME ANY WHERE * * Padma Vibhushan * * The Padma Vibhushan is the second-highest civilian award of the Republic of India , proceeded by Bharat Ratna and followed by Padma Bhushan . Instituted on 2 January 1954, the award is given for "exceptional and distinguished service", without distinction of race, occupation & position. Year Recipient Field State / Country Satyendra Nath Bose Literature & Education West Bengal Nandalal Bose Arts West Bengal Zakir Husain Public Affairs Andhra Pradesh 1954 Balasaheb Gangadhar Kher Public Affairs Maharashtra V. K. Krishna Menon Public Affairs Kerala Jigme Dorji Wangchuck Public Affairs Bhutan Dhondo Keshav Karve Literature & Education Maharashtra 1955 J. R. D. Tata Trade & Industry Maharashtra Fazal Ali Public Affairs Bihar 1956 Jankibai Bajaj Social Work Madhya Pradesh Chandulal Madhavlal Trivedi Public Affairs Madhya Pradesh Ghanshyam Das Birla Trade & Industry Rajashtan 1957 Sri Prakasa Public Affairs Andhra Pradesh M. C. Setalvad Public Affairs Maharashtra John Mathai Literature & Education Kerala 1959 Gaganvihari Lallubhai Mehta Social Work Maharashtra Radhabinod Pal Public Affairs West Bengal 1960 Naryana Raghvan Pillai Public Affairs Tamil Nadu H. V. R. Iyengar Civil Service Tamil Nadu 1962 Padmaja Naidu Public Affairs Andhra Pradesh Vijaya Lakshmi Pandit Civil Service Uttar Pradesh A. Lakshmanaswami Mudaliar Medicine Tamil Nadu 1963 Hari Vinayak Pataskar Public Affairs Maharashtra Suniti Kumar Chatterji Literature -

Osmania Medical College.Pdf
Osmania Medical College. The inception of this college was in 1946. Located in Hyderabad, Andhra Pradesh, the college is affiliated to NTR University of Health Sciences, while it was previously affiliated to the Osmania University of Hyderabad. Osmania Medical College Hyderabad is the only educational institution in India (and possibly the world), where every medical specialty has a separate training hospital. Initially, the college was called the Nizam’s Medical School in 1846. Later in 1920, the medical school was transformed into a medical college. The present campus at Koti was opened in 1964. The following hospitals fulfill the role of teaching hospitals for Osmania Medical College. 1. Osmania General Hospital - a multi speciality Tertiary-care hospital with advanced training in every sub-speciality 2. Government Maternity Hospital, Sultan Bazaar Hospital - a tertiary care hospital for Obstetrics and Gynecology. 3. Niloufer Hospital - a tertiary care hospital for Obstetrics, Pediatrics, Neonatology, maternal-fetal Medicine. 4. Sir Ronald Ross Institute of Tropical and Communicable Diseases - Dr. Ross elucidated the life cycle of malarial parasite at a place near Begumpet Airport, where a Building exists today in his Name. In his honour, The Quarantine hospital was rechristened Sir Ronald Ross Institute. (He was awarded the Nobel Prize in Medicine for this work) 5. Modern Government Maternity Hospital, Petlaburz - a tertiary care Obstetrics and Gynecology Hospital 6. Mehdi Nawaz Jung Institute of Oncology. 7. Sarojini Devi Eye Hospital - Tertiary care ophthalmological institute with advanced training in Ophthalmology 8. Government E. N. T. Hospital - Tertiary care hospital for ENT disorders. 9. Institute of Mental Health, Erragadda 10. -

POPULATION BASED CANCER REGISTRY, HYDERABAD Nizam's
Population BASED CANCER REGISTRY, HYDERABAD Nizam’s Institute of Medical Sciences Dr Sadashivudu Gundeti, MD, DM Principal Investigator, Associate Professor, Department of Medical Oncology Introduction PBCR Hyderabad was started on 1st September 2013 at Nizam’s Institute of Medical Sciences with the goal of finding out various types of cancers and their incidence rates in Hyderabad. Hyderabad is the capital city of newly formed Telangana state and is now the joint capital of Andhra Pradesh and Telangana. The Population Based Cancer Registry (PBCR) Hyderabad covers 217 sq.km with the population of 39,43,323 (2011 census) with males 20,18,575 (51%) and females 19,24,748 (49%). Most of the Population is engaged in Government sectors, Information Technology, Trade and Commerce. Sources covered for registry operations: Government order (G.O. No: 75) by the Principal Secretary, Government of Andhra Pradesh was circulated on 2nd May 2014 to all the health authorities in the District. Based on the government order we approached the government and private hospitals to start the data abstraction for PBCR Hyderabad. Data is being collected from all the source hospitals, Pathology labs etc. The major data sources are Nizam’s Institute of Medical Sciences (NIMS), Mehdi Nawaz Jung Institute of Oncology (MNJIO-RCC), Indo American Cancer Hospital and Research Institute (IACC) and Yashoda Group of Hospitals etc. Methodology to obtain Incidence Data Government OP Block, Pathology, Radiotherapy, Labs, Investigations and Hospitals Medical Record Room Reports Medical Records, Labs, Investigations and Private Hospitals Pathology Reports Reports are collected Patients are referred to source Clinical Labs from labs Hospitals Mortality Data Collection All cause Mortality Data are collected from Department of Births and Deaths, Greater Hyderabad Municipal Corporation (GHMC) and the cancer deaths are shortlisted, compiled and matched with the incidence data through proper coordination with the NCRP-Bangalore. -

Volume II) January, 2008 to December, 2012
An Overview of International Collaborative Research Projects in Health Research approved by Health Ministry’s Screening Committee (Volume II) January, 2008 to December, 2012 International Health Division Indian Council of Medical Research Published by Director-General Indian Council of Medical Research New Delhi-110029 June, 2013 The data compiled in the document corresponds to international collaborative projects approved by HMSC during January, 2008 to December, 2012 (5 years) ©Indian Council of Medical Research, New Delhi ISBN- 978-81-910091-4-9 Conceptualization, Design & Layout Mukesh Kumar, Harpreet Sandhu, Payal Prakash, Abhay Shankar Pandey Production Controller J. N. Mathur, New Delhi Printed at Dated the 26th April, 2013 Foreword The International Health Division of ICMR is publishing the second volume entitled “An Overview of International Collaborative Research Projects in Health Research approved by Health Ministry’s Screening Committee (HMSC)” during January, 2008 to December, 2012. I am pleased to share this document with you as a sequel of our previous publication entitled “An overview of international collaboration in biomedical research” which provided the information on the international collaborative research projects approved by Health Ministry’s Screening Committee (HMSC) during the years 2000 to 2007. The earlier document released in June 2008 was considered as a useful publication by the scientific community for providing a wealth of information especially relevant to those engaged in carrying out and promoting international collaboration through joint research projects. The current document provides ICMR’s strengths in health research for collaboration with different countries. It contains relevant information on various MoUs signed with different countries/ agencies and related details on the areas of interest and modes of collaboration. -
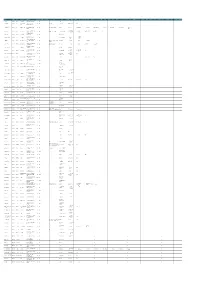
Srno Party Credit Grantor State Credit Grantor Branch Registered Address Outstanding Amount in Lacs Asset Classification Date Of
OUTSTANDING SRNO PARTY CREDIT GRANTOR STATE CREDIT GRANTOR BRANCH REGISTERED ADDRESS ASSET CLASSIFICATION DATE OF CLASSIFICATION OTHER BANK DIRECTOR 1 DIN FOR DIRECTOR 1 DIRECTOR 2 DIN FOR DIRECTOR 2 DIRECTOR 3 DIN FOR DIRECTOR 3 DIRECTOR 4 DIN FOR DIRECTOR 4 DIRECTOR 5 DIN FOR DIRECTOR 5 DIRECTOR 6 DIN FOR DIRECTOR 6 DIRECTOR 7 DIN FOR DIRECTOR 7 DIRECTOR 8 DIN FOR DIRECTOR 8 DIRECTOR 9 DIN FOR DIRECTOR 9 DIRECTOR 10 DIN FOR DIRECTOR 10 DIRECTOR 11 DIN FOR DIRECTOR 11 DIRECTOR 12 DIN FOR DIRECTOR 12 DIRECTOR 13 DIN FOR DIRECTOR 13 DIRECTOR 14 DIN FOR DIRECTOR 14 DIN FOR DIRECTOR 14 AMOUNT IN LACS 1 A & J IMPEX ALLAHABAD BANK PUNJAB ARMB CHANDIGARH E 124, phase -IV Focal point Ludhiana - 141002 1394 SUIT _ ANJU JAIN Survey No. 117, Kalgidhar Textile Mill Compound,Nr AHMEDABAD SARDAR PATEL 2 A A FABTEX PVT.LTD ALLAHABAD BANK GUJRAT Kashiram Mill, Hotel Good Luck lane, Narol, 1560 SUIT Bank of Maharashtra Annmol B. Aggarwala 1259880 Shrikant B. Aggarwala 2204753 NAGAR Ahmadabad. â €“ 382405 E-3, MANGOLPURI INDUSTRIAL AREA, PHASE-II, JAYANT MOHANLAL 3 ALLIED PERFUMERS PVT LTD. ALLAHABAD BANK DELHI NEW DELHI INTERNATIONAL 4402 SUIT LEAD BANK: BANK OF BARODA, SBBJ,SBT SANJAY JAIN 26147 RAJIV JAIN 26004 RAMESH SAREEN SANJEEV AGARWAL 25998 KAMAL KANT SHARMA ROHIT CHOWDHARY 26031 MOHAN GUPTA KAMAL KISHORE GUPTA DELHI-110034 GANDHI Reg. Office at 190, Poonamalle High Road, Chennai- Dr. Chandra Ravindran V R MEHTA PAN SUDHIR CHANDRA PAN C M K REDDY PAN 4 Arvind Remedies Ltd ALLAHABAD BANK TAMIL NADU GEORGE TOWN CHENNAI 14306 SUIT PNB, UBI, SBI, IDBI, KVB, IOB, CORPORATION Dr. -
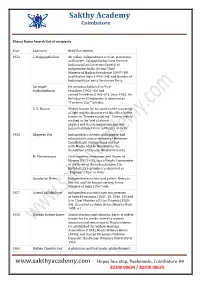
Sakthy Academy Coimbatore
Sakthy Academy Coimbatore Bharat Ratna Award: List of recipients Year Laureates Brief Description 1954 C. Rajagopalachari An Indian independence activist, statesman, and lawyer, Rajagopalachari was the only Indian and last Governor-General of independent India. He was Chief Minister of Madras Presidency (1937–39) and Madras State (1952–54); and founder of Indian political party Swatantra Party. Sarvepalli He served as India's first Vice- Radhakrishnan President (1952–62) and second President (1962–67). Since 1962, his birthday on 5 September is observed as "Teachers' Day" in India. C. V. Raman Widely known for his work on the scattering of light and the discovery of the effect, better known as "Raman scattering", Raman mainly worked in the field of atomic physics and electromagnetism and was presented Nobel Prize in Physics in 1930. 1955 Bhagwan Das Independence activist, philosopher, and educationist, and co-founder of Mahatma Gandhi Kashi Vidyapithand worked with Madan Mohan Malaviya for the foundation of Banaras Hindu University. M. Visvesvaraya Civil engineer, statesman, and Diwan of Mysore (1912–18), was a Knight Commander of the Order of the Indian Empire. His birthday, 15 September, is observed as "Engineer's Day" in India. Jawaharlal Nehru Independence activist and author, Nehru is the first and the longest-serving Prime Minister of India (1947–64). 1957 Govind Ballabh Pant Independence activist Pant was premier of United Provinces (1937–39, 1946–50) and first Chief Minister of Uttar Pradesh (1950– 54). He served as Union Home Minister from 1955–61. 1958 Dhondo Keshav Karve Social reformer and educator, Karve is widely known for his works related to woman education and remarriage of Hindu widows. -

Final Annual Report.Cdr
60 years of Insurance Education & Training I N S U R A N C E I N S T I T U T E O F I N D I A PLOT NO. C-46, G-BLOCK, BANDRA - KURLA COMPLEX, BANDRA (EAST), MUMBAI - 400 051 Diamond Jubilee [1955 - 2015] OBJECTIVES To run College and conduct examinations, oral and written, in insurance theory and practice and related subjects for awarding certificates, diplomas and degrees to those interested in insurance. To give oral and postal tuitions, prepare and supply reading materials and similar other educative methods for encouraging and assisting the study of any subject bearing on any branch of insurance. To offer scholarships, grants and prizes for research or any other educational work bearing on insurance. To ascertain the law and practice relating to all matters connected with insurance and to disseminate such knowledge among those interested in insurance. The activities and programmes of the institute, among others, assist people in the insurance industry, to acquire the skills and expertise to meet the growing needs of multiplicity of customers - the objective being to enhance professional insurance service to the millions in this country. th 58 Annual Report & Accounts 2013-14 CONTENTS Sr. Page No. No. 14 18 30 56 59 61 64 67 72 1 60 years of Insurance Education & Training I N S U R A N C E I N S T I T U T E O F I N D I A Diamond Jubilee [1955 - 2015] PAST PRESIDENTS & PAST SECRETARIES / SECRETARY-GENERALS C.R.C. Gardiner, O.B.E.,J.P.,F.I.I.I. -

Bid/Issue Opens on July 29, 2004 • Bid/Issue Closes on August 5, 2004
Registered Office: Bombay House, 24 Homi Mody Street, Fort, Mumbai 400 001, India. Tel.: (91 22) 5665 8282, Fax: (91 22) 5665 8080, Email: [email protected], Website: www.tcs.com Corporate Office: 11th Floor, Air India Building, Nariman Point, Mumbai 400 021, India. Tel.: (91 22) 5668 9999, Fax: (91 22) 5668 9661, Email:[email protected] Public Issue of 55,452,600 Equity Shares of Re. 1 each for cash at a price of Rs.[•] per Equity Share aggregating Rs.[•] million, consisting of a Fresh Issue of 22,775,000 Equity Shares of Re. 1 each by Tata Consultancy Services Limited (“TCS Limited” or the “Company” or the “Issuer”) and an Offer for Sale of 32,677,600 Equity Shares by Tata Sons Limited (“Tata Sons”) and certain other shareholders of TCS Limited (together with Tata Sons, the “Selling Shareholders”). The Fresh Issue and the Offer for Sale are jointly referred to herein as the “Offer”. The Offer will include Employee Reservation Portion of 5,545,260 Equity Shares. There will also be a Green Shoe Option of 8,317,880 Equity Shares of Re. 1 each to be offered by Tata Sons for cash at a price of Rs. [•] per Equity Share aggregating Rs. [•] million. The Offer and the Green Shoe Option aggregate Rs. [•] million. The face value of the Equity Shares is Re. 1 and the Offer Price is [•] times of the face value. 100% BOOK BUILDING ISSUE BID/ISSUE OPENS ON JULY 29, 2004 BID/ISSUE CLOSES ON AUGUST 5, 2004 QIBs Non-Institutional Investors Retail Individual Investors QIBs Non-Institutional Investors Retail Individual Investors (1) (1) Allotment Mode -

Insurance Institute of India Annual Report
INSURANCE INSTITUTE OF INDIA 60th Annual Report & Accounts 2015 - 16 OBJECTIVES To run College and conduct examinations, oral and written, in insurance theory and practice and related subjects for awarding certificates, diplomas and degrees to those interested in insurance. To give oral and postal tuitions, prepare and supply reading materials and similar other educative methods for encouraging and assisting the study of any subject bearing on any branch of insurance. To offer scholarships, grants and prizes for research or any other educational work bearing on insurance. To ascertain the law and practice relating to all matters connected with insurance and to disseminate such knowledge among those interested in insurance. The activities and programmes of the Institute, among others, assist people in the insurance industry, to acquire the skills and expertise to meet the growing needs of multiplicity of customers – the objective being to enhance professional insurance service to the millions in this country. 1 CONTENTS Sr. Page No. No. 1. Past Presidents & Past Secretaries / Secretary-Generals________________________________ 2 2. The Institute ___________________________________________________________________3 3. Officers of the Institute / College of Insurance _________________________________________5 4. Members of Various Committee ___________________________________________________6 5. Report to the Council ___________________________________________________________14 6. Report of Board of Education_____________________________________________________19