Directory of Services
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Application Form for Ug / Pg Courses the Sankara Nethralaya Academy
THE SANKARA NETHRALAYA ACADEMY (Unit of Medical Research Foundation) www.thesnacademy.ac.in City / Hospital Campus Vanagaram / Academic Campus New No.41, Old No:18, College Road, Nungambakkam, No.9, Vanagaram – Ambattur Road, Ayanambakkam, Chennai – 600 006. Ph No. 044 – 42271500 Chennai – 600 095. Ph: 044 4908 6000 E-mail: [email protected] APPLICATION FORM FOR UG / PG COURSES Registration Details: (To be filled by TSNA official) Registration Date: Registration No: Student’s Recent Remarks: Registration –in-charge (Sign) Colour Photograph Course Applied for Name of the Applicant with initials (as in +2 Mark Sheet – in BLOCK letters) Expansion of Initials Gender (Please tick) Male Female Neutral Place of Birth Date of Birth D D M M Y Y Y Y Nationality Religion Community Address for Communication Blood Group Pin Code E-mail ID Phone with STD Code Mobile No Aadhaar (UAID) No Parent Name Name of Guardian (If student not staying with parents) Parent/Guardian Address for Communication (If different from above) Pin Code E-mail ID Phone with STD Code Mobile No Details of Educational Qualifications Month & Major Name of the School / Aggregate Course Studied Year of Medium Subjects College / University % Marks /Class Passing SSLC/ 10th Std Hr. Sc. /12th Std Under Graduate Post Graduate (Enclose Attested copies of SSLC/Hr. Secondary certificates and UG Provisional Certificates or Degree Certificates.) Transfer Certificate Details (Mandatory for all courses) Certificate No Date of Issue Issuing Institution Issuing Authority Eligibility Certificate -
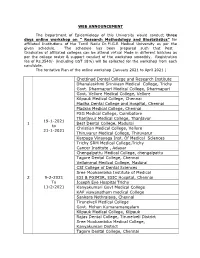
Research Methodology and Biostatistics” for Affiliated Institutions of the Tamil Nadu Dr.M.G.R Medical University As Per the Given Schedule
WEB ANNOUNCEMENT The Department of Epidemiology of this University would conduct three days online workshop on “ Research Methodology and Biostatistics” for affiliated Institutions of the Tamil Nadu Dr.M.G.R Medical University as per the given schedule. The schedule has been prepared such that Post Graduates of affiliated colleges can be attend virtual Mode in different batches as per the college roster & support conduct of the workshop smoothly. Registration fee of Rs.3540/- (including GST 18%) will be collected for the workshop from each candidate. The tentative Plan of the online workshop (January 2021 to April 2021 ) Chettinad Dental College and Research Institute Dhanalaskhmi Srinivasn Medical College, Trichy Govt. Dharmapuri Medical College, Dharmapuri Govt. Vellore Medical College, Vellore Kilpauk Medical College, Chennai Madha Dental College and Hospital, Chennai Madras Medical College, Chennai PSG Medical College, Coimbatore Thanjavur Medical College, Thanjavur 19-1-2021 1 Best Dental College, Madurai to Christian Medical College, Vellore 21-1-2021 Thiruvarur Medical College, Thiruvarur Karpaga Vinayaga Inst. Of Medical Sciences Trichy SRM Medical College,Trichy Cancer Institute , Adayar Chengalpattu Medical College, chengalpattu Tagore Dental College, Chennai Vellammal Medical College, Madurai CSI College of Dental Sciences Sree Mookambika Institute of Medical 29-2-2021 ESI & PGIMSR, ESIC Hospital, Chennai To Joseph Eye Hospital Trichy 11-2-2021 Kanyakumari Govt Medical College KAP viswanatham medical College Sankara Nethralaya, Chennai Tirunelveli Medical College Govt. Mohan Kumaramangalam Kilpauk Medical College, Kilpauk Rajas Dental College, Tirunelveli District Sree Mookambika Medical College, Kanyakumari District Tagore Dental College, Chennai Trichy SRM Medical College,Trichy Vivekanandha Dental College for Women, Namakkal Christian medical College, Vellore Govt. -

“Best Hospital in Ophthalmology” for Year 2014 Eyelights February 2015
eyelights NABL Accreditation NABH Accreditation for Sankara Nethralaya Main Number : 68 February 2015 SN confers high SN’s devout Keracon 2014 Nobility honours on its call to defy Cornea Society through 36th 3 Diabetic 4 6 7 of India’s 2nd Charity Foundation Day Retinopathy annual meet Sankara Nethralaya confers high honours on its 36th Foundation Day The Week- Nielsen survey declares Sankara Nethralaya as the “Best Hospital in Ophthalmology” for year 2014 eyelights February 2015 R e c o g n i t i o n s & A w a r d s “Best Hospital in A research paper on the molecular diagnostic tests PCRs for rapid Ophthalmology” diagnosis of tuberculosis, from Sankara Nethralaya is adjudged as for year 2014 by the best published paper in bacteriology. The Week - Nielsen The highly prestigious “The The research paper by Dr. K.Lily Therese, HOD & Sr. Prof. L&T Microbiology Week-Nielsen” survey for Research Centre, KNBIRVO was adjudged as the best paper in bacteriology rating the best hospitals in published in the Indian Journal of Medical Microbiology for year 2013, at the the country has declared XXXVIIIth National Conference of the IAMM held at SMS Medical College, Jaipur Sankara Nethralaya as the between 17th and 19th October 2014 and the presenter was honoured with a Medal. “ B e s t H o s p i t a l i n Sankara Nethralaya Academy students bag merit Ophthalmology” once again. The survey was scholarships from TechMed Healthcare, Chennai conducted on stringent Techmed healthcare organized a state level q u a l i t y m e a s u r e s b y quiz competition for the “LIFE SCIENCE experts, covering 15 cities AWARD, 2014” on 18th October, 2014. -

Sankara Nethralaya Salutes an Old Friend and Supporter on His Birth Centenary an Institution Dedicated to Service Honours a Life
eyelights NABL Accreditation NABH Accreditation for Sankara Nethralaya Main Number : 77 April 2019 Eye donation Awards SN Academy Stalwarts recall awareness conducts & greatness 2 creation 4 extensive 7 Recognitions 6 of Iravatham drive health check Mahadevan An institution dedicated to Service honours a lifelong dedication to Service Sankara Nethralaya salutes an old friend and supporter on his birth centenary eyelights April 2019 R e c o g n i t i o n s & A w a r d s Best Hospital A focussed study into the niche visual needs and challenges in Ophthalmology of the men who ensure our safety and comfort while driving / riding wins high recognition at major topical meet Dr Rashima Asokan, Assistant Professor, ESO, researcher in optometry and Member - 'Indian Association of Occupational health' was awarded the 'Golden Jubilee Medal' for her most insightful paper in the field of occupational optometry, titled 'Impact of Vision correction on the quality of life and productivity among automobile mechanics' at the 67th State meeting of the Indian Association of Occupational Health (IAOH) TN chapter. Senior Research Optometrist, Sankara Nethralaya awarded Sankara Nethralaya was with a Doctorate by SASTRA University adjudged as the 'Best Ms Kalpa Negiloni, Senior Research Optometrist, Sankara Nethralaya was H o s p i t a l i n awarded with a Doctorate by SASTRA University for her trailblazing Ophthalmology' at the finding and recommendation on the lighting and seating arrangement in 'Mayan Awards- 2018' held school classrooms, titled “Environmental factors and visual in January 2019. The award requirements in school classrooms and development of was presented by Mrs Kiran recommendation for classroom visual environment”. -

List of Board of Governors
23.09.2013 The Sankara Nethralaya Academy Board of Governors list S No Name Designation E - Mail 1 Dr A Kalanidhi Vice-Chairman – CSTAR, [email protected] Former Vice Chancellor, Anna University, Chennai 2 Dr Ashok Jhunjhunwala Chairman & Professor - Electrical Engineering [email protected] Department, IIT Madras 3 Dr Lingam Gopal Board Member, Medical Research Foundation, [email protected] Chennai 4 Dr Neil Miller Professor of Ophthalmology, Neurology, and [email protected] Neurosurgery at the Johns Hopkins Medical Institutions 5 Dr Ravindra D Bapat Former Vice-Chancellor of Mahatma Gandhi [email protected] Mission University of Health Sciences 6 Dr S Bhaskaran Chairman,Medical Research Foundation, [email protected] Chennai 7 Dr S Swaminathan Director, Centre for Nanotechnology & [email protected] Advanced Biomaterials.SASTRA University,Thanjavur 8 Dr T K Parthasarathy Pro Chancellor -Sri Ramachandra [email protected] University,Chennai 9 Dr Vasan K S Managing Director, Medical Research [email protected] Foundation, Chennai 10 Dr Vijay Dasmania Vice Chancellor – HIHT University,(The [email protected] Himalayan Institute Hospital Trust), Dehradun, Uttaranchal 11 Dr S Meenakshi Director – Academics, Medical Research [email protected] Foundation, Chennai 12 Dr. S. S. Badrinath Chairman Emeritus & President, Medical [email protected] Research Foundation, Chennai 13 Lion Dr Chandrasekara Vice Chancellor-Sri Devaraj Urs Academy of [email protected] Shetty Higher Education and Research, Karnataka 14 Lion P Haridas Senior Advocate & Secretary [email protected] D G Vaishnav College, Chennai 15 Mr D R Karthikeyan Advisor: Law-Human Responsibilities- [email protected] Corporate Affairs 16 Mr Homi Bhabha Director – Mercl Ltd,& Ceekay Daikin Ltd [email protected] 17 Mr MS Jayaraman Management Consultant & Coach (Leadership [email protected] building), Atheros, Chennai. -

Chennai PPN Network Hospital List Sr
Chennai PPN Network Hospital List Sr. Tel_are Hospital Name Location Address Pin_No Tel_No MobileNo City State E_Mail PPN City No. a_code 81-86 Annai Valasaravakk Tamil [email protected] 1 A N N Hospital Therasa Street 600087 044 24869300 9442360800 Chennai Chennai am Nadu om Indira Nagar # 172, SOLAIAPPAN STREET, NEAR drselvaraj@avhospital Tamil 2 A V Hospitals Parrys MAHARANI 600021 044 25955859 9444013879 Chennai s.com;kalaivani.vetrise Chennai Nadu THEATRE, [email protected] MANNADY [email protected] No. 395, T H Road, Tamil m;aakashsrk_dr@yah 3 Aakash Hospital Thiruvotriyur Near Thiruvottriyur 600019 044 25730099 9444382293 Chennai Chennai Nadu oo.co.in;aakashsrkdr Bus Terminus @gmail.com abhijay.claims@gmail. 22/2, E.S.I Hospital Abhijay Hospital (p) Tamil com; 4 Perambur Road, Perambur, 600011 044 49015050 9884368589 Chennai Chennai Ltd Nadu cashless@abhijayhosp Peravallur itals.com adityahospital@gmail. 7, Barnaby Road, Tamil com; 5 Aditya Hospital Kilpauk 600010 044 26411447 9840727909 Chennai Chennai Kilpauk, Chennai Nadu insurance@adityahos pital.co.in insurance@agadaheal thcare.net;karthikaran No 8, Dr Nair Road, i.p@agadahealthcare. Agada Healt Care Tamil 6 T Nagar Behind Vani Mahal, 600017 044 28152604 9087718512 Chennai net;prabhub@agadah Chennai Pvt Ltd Nadu T Nagar ealthcare.net; operations@agadahea lthcare.net 1,Sowrastra Nungambakk Tamil sureshdrsuresh@yaho 7 Amma Hospital Nagar,7th 600094 044 24840441 9840048896 Chennai Chennai am Nadu o.co.in; Street,Choolaimedu new no 80 ,7 th ammayieyehospital@y Ammayi Eye Tamil -

Annual Report & Accounts 2015
MEDICAL RESEARCH FOUNDATION OLD NO.18, NEW NO.41, COLLEGE ROAD, CHENNAI 600 006 ANNUAL REPORT & ACCOUNTS 2015 - 2016 1 MEDICAL RESEARCH FOUNDATION To The Members of the Medical Research Foundation 29th August 2016 NOTICE NOTICE IS HEREBY GIVEN TO THE MEMBERS OF THE MEDICAL RESEARCH FOUNDATION THAT THE 38TH ANNUAL GENERAL MEETING OF THE FOUNDATION WILL BE HELD ON SATURDAY, THE 24th SEPTEMBER 2016 AT 5.00 P.M. AT BOARD ROOM, 2ND FLOOR, KAMALNAYAN BAJAJ RESEARCH CENTRE BLOCK, NEW NO 41, OLD NO 18, COLLEGE ROAD, CHENNAI 600 006. AGENDA 1. To consider and adopt the Audited Income and Expenditure Account for the year ended March 31, 2016 and the Balance Sheet as at that date together with the Report of the Auditors thereon. 2. To consider and adopt the Annual report of the Foundation for the year 2015-16. 3. To appoint Auditors and to fix their remuneration for the year ending 31st March 2017. 4. To elect Members to the Board of Management in the vacancies caused by the retirement of the following Members, who being eligible offer themselves for re-appointment Sl. No Name Category of Membership 1 Mr. C. Ramakrishna Founder member 2 Dr. M. Saravanan Donor member 3 Mr. V. Govind Donor member 4 Mr. N. Ranganatha Gupta Donor member 5 Mr. Surendra M Mehta Donor member 6 Mr. N. Sugalchand Jain Donor member 7 Mr. R.C. Parekh Donor member 8 Dr. Usha Barwale Zehr Donor member 9 Mr. G. Ramachandran Donor member 10 Dr. S. S. Badrinath Honorary member 11 Dr. -

LIST of EMPANELLED HOSPITALS Regional Centre City Name
LIST OF EMPANELLED HOSPITALS Regional Centre City Name of Hospital/Diag Address Phone/Mob/Email Approved Date of MOA Vaild Recognized for Status of hospital Status of hospital nostic/Dental Centre by MoD Signing up to as per MoA as per Govt letter MOA 373 CHENNAI Chennai Sankara Nethralaya (Unit of 18, College Road, Mob: 9380699128, [email protected] 29-Oct-04 03-Feb-15 19-Mar-16 Ophthalmology and Ophthalmic Emergency. Ophthalmology. NABH NON NABH Medical Research Foundation) Chennai 600 006 374 CHENNAI Chennai Madras Medical Mission 4A Dr JJ Nagar Mogappair Phone 044 26565960, 26565961 06-Aug-03 09-Mar-12 08-Mar-14 -- Surgery Cardio- Thoracic Surgery NON NABH NON NABH Chennai 600 037 Medicine Cardiology and Interventional Cardiology. 375 CHENNAI Chennai Ehrlich Laboratory Pvt Ltd. 46 & 48 Masilamani Road, PH 04428130514, 28130460 06-Aug-03 04-Oct-13 14-Jul-15 Microbiology, Pathology, Radio-Diagnosis (incl USG) Others (Specify) : TMT, Echo-Cardiography. NON NABH NON NABH Balaji Nagar Royapettah Chennai Pin 600014 376 CHENNAI Chennai SRL Limited AB-46, 1st Street, 6th Main 06-Oct-15 Clinical Biochemistry, Clinical Pathology, Haematology & NABL NABL Road, Anna Nagar, Chennai, Immunohaematology, Microbiology & Serology, Cytopathology Tamil Nadu 377 CHENNAI Sivanandapuram Sri Kanchi Kamakoti Medical Sathy Road, 20-Jun-14 22-Aug-14 21-Aug-16 General Services : Ophthalmology (Refraction, Cataract NON NABH NON NABH , Coimbatore Trust Sankara Eye Centre Sivananathapuram, Surgeries with IOL Implantations). Saravanampatty, Coimbatore – 641035 Specialised Services – Cataract/Glaucoma, Retinal – Medical – Vitreo – Retinal Surgery, Strabismus and Occuloplasty & Adnexa & other Specialised treatment. 378 CHENNAI Dindigul City Hospital, 4/361, Gandhi Ji Nagar, 9360930003, 22-Nov-06 01-Aug-14 31-Jul-16 General Medicine, ENT, Orthopedics, Dental (including oral Surgery Plastic and Reconstructive Surgery and NON NABH NON NABH Pavalam Trauma Centre, Trichy Road Dindigul [email protected] surgery), Microbiology, General Surgery, Ophthalmology, Laparoscopic Surgery. -

BE BIOMEDICAL ENGINEERING Professor-In-Charge
BIOMEDICAL ASSOCIATION CAREER GUIDANCE It is an association organized by the final Biomedical engineers design instruments, year Biomedical students with the devices and software used in healthcare, guidance of faculty members. Various develop new procedures using knowledge events such as symposium, workshops, from many technical sources or conduct career guidance programs, technical quiz research needed to solve clinical competitions are being conducted by this problems. association in order to guide, enlighten and inspire students regarding the field of Biomedical Engineering. B.E BIOMEDICAL ENGINEERING Professor-in-charge: Prof.Dr.M.Sasikala STUDENT ACHIEVEMENTS Kurukshetra Project CTDT Project Top 3 innovations at Living Talent, Dubai Intel Innovation challenge 2018, Bangalore Biospectra is a National level technical Intern at Taipei Medical University, symposium conducted every year by the Taiwan. biomedical engineering students. Here FTTP, Swinburne University, Australia. Intra and Intercollege students can Student exchange program at participate in this symposium university of Bern, Switzerland. Top 1 Innovator of India under Biomedical Research-18, HITCON, Ahmedabad. FOR FURTHER INFORMATION PLEASE CONTACT Dr. M. Meenakshi, Head of the Department Department of Electronics and Communication Engineering, College of Engineering Guindy, Anna University, Chennai-600 025, India. Phone: 044 22358880/22358882 E-mail : [email protected] VISION ELIGIBILITY AREAS OF STUDY HOSPITAL TRAINING To be recognized as a benchmark and Candidate -

Medical Research Foundation Notice
MEDICAL RESEARCH FOUNDATION To The Members of the Medical Research Foundation 7th September 2011 NOTICE NOTICE IS HEREBY GIVEN TO THE MEMBERS OF THE MEDICAL RESEARCH FOUNDATION THAT THE 33RD ANNUAL GENERAL MEETING OF THE FOUNDATION WILL BE HELD ON THRUSDAY, THE 29th SEPTEMBER 2011 AT 6.00 P.M. AT BOARD ROOM, 2ND FLOOR, KAMALNAYAN BAJAJ RESEARCH CENTRE BLOCK, NEW NO 41, OLD NO 18, COLLEGE ROAD, CHENNAI 600 006. THE AGENDA FOR THE MEETING IS GIVEN BELOW: AGENDA 1. To consider and adopt the Annual report of the Foundation for the year 2010-11. 2. To consider and adopt the Audited Income and Expenditure Account for the year ended March 31, 2011 and the Balance Sheet as at that date together with the Report of the Auditors thereon. 3. To appoint Auditors and to fix their remuneration for the year ending 31st March 2012. 4. To elect Members to the Board of Management in the vacancies caused by the retirement of the following Members, who are eligible for re-appointment. Name Category Mr. H D Malesra Patron Member Mr.Mani S Subramonian Patron Member 5. To elect Members to the Board of Management in the existing vacancy. Kindly make it convenient to attend the meeting. N.SUGALCHAND JAIN HONY. SECRETARY & TREASURER 1 MEDICAL RESEARCH FOUNDATION SANKARA NETHRALAYA MISSION STATEMENT QUALITY OBJECTIVES The mission of Sankara Nethralaya is to To maintain the quality of ophthalmic provide Total Eye-care solutions of highest s e r v i c e s i n a c c o r d a n c e w i t h standards to all sections of community international standards. -
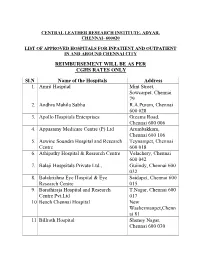
REIMBURSEMENT WILL BE AS PER CGHS RATES ONLY Sl.N Name Of
CENTRAL LEATHER RESEARCH INSTITUTE, ADYAR, CHENNAI- 600020 LIST OF APPROVED HOSPITALS FOR INPATIENT AND OUTPATIENT IN AND AROUND CHENNAI CITY REIMBURSEMENT WILL BE AS PER CGHS RATES ONLY Sl.N Name of the Hospitals Address 1. Amrit Hospital Mint Street, Sowcarpet, Chennai 79 2. Andhra Mahila Sabha R.A.Puram, Chennai 600 028 3. Apollo Hospitals Enterprises Greams Road, Chennai 600 006 4. Appasamy Medicare Centre (P) Ltd Arumbakkam, Chennai 600 106 5. Aswine Soundra Hospital and Research Teynampet, Chennai Centre 600 018 6. Athipathy Hospital & Research Centre Velachery, Chennai 600 042 7. Balaji Hospsitals Private Ltd., Guiindy, Chennai 600 032 8. Balakrishna Eye Hospital & Eye Saidapet, Chennai 600 Research Centre 015 9. Barathiraja Hospital and Research T.Nagar, Chennai 600 Centre Pvt.Ltd 017 10. Beach Chennai Hospital New Washermanpet,Chenn ai 81 11. Billroth Hospital Shenoy Nagar, Chennai 600 030 12. BSS Hospital Mandaveli, Chennai 600 028 13. Cancer Institute (WIA) Adyar, Chennai 600 020 14. Chettinad Health City Kelambakkam, Chennai 15. Child Trust Hospital Nungambakkam, 600 034 16. City Hospital Adyar, Chennai 600 020 17. CSI Kalyani General Hospital Dr. R.K.Salai, Chennai 600 004 18. CSI Rainy Multi Speciality Hospital 45 GA Road, Chennai 600 021 19. Deepam Hospitals (P) Ltd., West Tambaram, Chennai 600 045 20. Devaki Hospital Ltd Mylapore, Chennai 600 004 21. Dr. Agarwa l Eye Hospital Cathedral Road, Chennai 600 086 22. Dr. Meenakshi Hospitals Avadi, Chennai 600 054 23. Dr. Rabindran’s Health Care Centre Pvt. Ambattur, Chennai Ltd., 600 053 24. Dr. Rai Memorial Medical Centre Anna Salai, Chennai 600 018 25. -

Palanivelu Mahesh Shanmugam Graduated from Stanley Medical College, Madras in 1990 with a Gold Medal for Proficiency in Medicine
Dr. Palanivelu Mahesh Shanmugam graduated from Stanley Medical College, Madras in 1990 with a gold medal for proficiency in medicine. He joined Sankara Nethralaya, Chennai and completed Diploma in Ophthalmology (University of Madras) in 1992, fellowship in vitreoretinal diseases (Sankara Nethralaya, Chennai) in 1993, FRCS Edinburgh in 2002 and PhD in Ophthalmology (Dr.MGR Medical University, Chennai) in 2003. He continued at Sankara Nethralaya after post-graduation to become the Associate Director of ocular oncology and Senior Consultant, department of vitreoretinal diseases until 2005. He has worked as Associate Professor at The Chinese University of Hong Kong and as Honorary Professor, Shantou University, PRC. He specialises in medical and surgical management of vitreoretinal diseases and ocular oncology. He has more than 110 publications in peer-reviewed journals and 15 chapters in textbooks on vitreoretinal disorders, diseases and surgery, ocular oncology. He has co-developed the technique of optimized vacuum control in vitrectomy equipment, which is pending patent approval. He has been awarded the Swarnalatha Punshi award for the Best Research worker by Sankara Nethralaya, Col. Rangachari award by the All India Ophthalmological Society, the Distinguished ophthalmologist Gold Medal by the Uttarakhand ophthalmological society and the Siva Reddy award by the Andhra Pradesh Ophthalmological Society. He is a section editor, ocular oncology for the Indian Journal of Ophthalmology and has served as a reviewer for Ophthalmology, British Journal of Ophthalmology, Eye, Clinical and Experimental Ophthalmology, Retinal Cases & Brief Reports, JAAPOS, BMC ophthalmology, Indian Journal of Ophthalmology, Journal of Ophthalmology, Journal of medical case reports, Indian journal of Neurology, International journal of biomedical and clinical engineering, Current drug metabolism.