Corydoras Diphyes (Siluriformes: Callichthyidae) and Otocinclus Mimulus (Siluriformes: Loricariidae), Two New Species of Catfishes from Paraguay, a Case of …
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Diversion in Brazil Threatens Biodiversit
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/332470352 Water diversion in Brazil threatens biodiversity Article in AMBIO A Journal of the Human Environment · April 2019 DOI: 10.1007/s13280-019-01189-8 CITATIONS READS 0 992 12 authors, including: Vanessa Daga Valter Monteiro de Azevedo-Santos Universidade Federal do Paraná 34 PUBLICATIONS 374 CITATIONS 17 PUBLICATIONS 248 CITATIONS SEE PROFILE SEE PROFILE Fernando Pelicice Philip Fearnside Universidade Federal de Tocantins Instituto Nacional de Pesquisas da Amazônia 68 PUBLICATIONS 2,890 CITATIONS 612 PUBLICATIONS 20,906 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Freshwater microscrustaceans from continental Ecuador and Galápagos Islands: Integrative taxonomy and ecology View project Conservation policy View project All content following this page was uploaded by Philip Fearnside on 11 May 2019. The user has requested enhancement of the downloaded file. The text that follows is a PREPRINT. O texto que segue é um PREPRINT. Please cite as: Favor citar como: Daga, Vanessa S.; Valter M. Azevedo- Santos, Fernando M. Pelicice, Philip M. Fearnside, Gilmar Perbiche-Neves, Lucas R. P. Paschoal, Daniel C. Cavallari, José Erickson, Ana M. C. Ruocco, Igor Oliveira, André A. Padial & Jean R. S. Vitule. 2019. Water diversion in Brazil threatens biodiversity: Potential problems and alternatives. Ambio https://doi.org/10.1007/s13280-019- 01189-8 . (online version published 27 April 2019) ISSN: 0044-7447 (print version) ISSN: 1654-7209 (electronic version) Copyright: Royal Swedish Academy of Sciences & Springer Science+Business Media B.V. -

Investigating the Feasibility to Remove Shp Pandeiros: Lessons from Fish Fauna
RAFAEL COUTO ROSA DE SOUZA INVESTIGATING THE FEASIBILITY TO REMOVE SHP PANDEIROS: LESSONS FROM FISH FAUNA LAVRAS-MG 2017 RAFAEL COUTO ROSA DE SOUZA INVESTIGATING THE FEASIBILITY TO REMOVE THE SHP PANDEIROS: LESSONS FROM FISH Tese apresentada à Universidade Federal de Lavras, como parte das exigências do Programa de Pós-Graduação em Ecologia Aplicada, para a obtenção do título de Doutor. Prof. Dr. Paulo dos Santos Pompeu Orientador LAVRAS-MG 2017 Ficha catalográfica elaborada pelo Sistema de Geração de Ficha Catalográfica da Biblioteca Universitária da UFLA, com dados informados pelo(a) próprio(a) autor(a). Souza, Rafael Couto Rosa. Investigating the feasibility to remove SHP Pandeiros: Lessons from fish fauna / Rafael Couto Rosa Souza. - 2017. 79 p. Orientador(a): Paulo Santos Pompeu. Tese (doutorado) - Universidade Federal de Lavras, 2017. Bibliografia. 1. Dam removal. 2. Fish ecology. 3. Trophic ecology. I. Pompeu, Paulo Santos. II. Título. O conteúdo desta obra é de responsabilidade do(a) autor(a) e de seu orientador(a). RAFAEL COUTO ROSA DE SOUZA INVESTIGATING THE FEASIBILITY TO REMOVE THE SHP PANDEIROS: LESSONS FROM FISH FAUNA Tese apresentada à Universidade Federal de Lavras, como parte das exigências do Programa de Pós-Graduação em Ecologia Aplicada, para a obtenção do título de Doutor. APROVADA em 04 agosto de 2017. Profa. Carla Rodrigues Ribas UFLA Prof. Marcos Callisto de Faria Pereira UFMG Prof. Rafael Pereira Leitão UFMG Prof. Luis Antônio Coimbra Borges UFLA Prof. Dr. Paulo dos Santos Pompeu Orientador LAVRAS-MG 2017 Dedico este trabalho aos peixes que sacrifiquei no intuito de conservar o ambiente no qual eles viviam, apesar de contraditório suas mortes não passaram desapercebidas. -

Diversidade E Conservação Dos Peixes Da Caatinga Ricardo Rosa Universidade Federal Da Paraíba
Diversidade e conservação dos peixes da Caatinga Ricardo Rosa Universidade Federal da Paraíba 149 Arq. Fundação Biodiversitas Arq. Fundação Açude de Cocorobó, Canudos - BA INTRODUÇÃO O conhecimento da diversidade e Velhas cujas distribuições se estendem para taxonomia de peixes de água doce áreas do bioma Caatinga na bacia do rio neotropicais é ainda incipiente (Menezes São Francisco. 1992, Rosa & Menezes 1996). Para as A Comissão Científica de Exploração, bacias interiores do Nordeste brasileiro, que constituída pelo Instituto Histórico e perfazem a maior parte dos ambientes Geográfico Brasileiro, efetuou coletas de peixes aquáticos do bioma Caatinga, essa de água doce no Ceará, entre os anos de 1859 situação é predominante. Os trabalhos de e 1861. Entretanto, os espécimes oriundos inventário ictiofaunístico nessa região, desse trabalho não foram adequadamente apesar de terem sido iniciados no século conservados no Museu Nacional (Braga 1962, XIX, são ainda escassos e localizados. Paiva & Campos 1995). A Expedição Thayer, organizada por Histórico e estado do conhecimento Louis Agassiz, que percorreu o Brasil entre os Johan von Spix e Karl von Martius, anos de 1865 e 1866, obteve espécimes de em sua expedição pelo Brasil, coletaram peixes provenientes das bacias dos rios São espécimes zoológicos durante os anos de Francisco, coletados por Orestes Saint-John 1818 e 1819 em diversas localidades do e John Allen, e Parnaíba, coletados por Orestes bioma Caatinga, nos estados da Bahia, Saint-John. Esses peixes foram depositados Pernambuco, Ceará, Piauí e Maranhão no Museum of Comparative Zoology, da (Papavero 1971, Paiva & Campos 1995). Universidade de Harvard, mas apenas uma Os peixes obtidos nessa expedição foram pequena parte do material foi trabalhada no posteriormente trabalhados por Spix e contexto de revisões sistemáticas e serviu para Agassiz (Selecta genera et species piscium a descrição de novas espécies de peixes do Brasiliensium, 1829-1831) (Paiva & Nordeste (p. -

Landscape and Habitat Characteristics Associated with Fish Occurrence and Richness in Southern Brazil Palustrine Wetland Systems
Environ Biol Fish DOI 10.1007/s10641-013-0152-4 Landscape and habitat characteristics associated with fish occurrence and richness in southern Brazil palustrine wetland systems Leonardo Maltchik & Luis Esteban Krause Lanés & Friedrich Wolfgang Keppeler & Ana Silvia Rolon & Cristina Stenert Received: 18 July 2012 /Accepted: 12 June 2013 # Springer Science+Business Media Dordrecht 2013 Abstract We investigated the influence of environ- The predictors of fish richness were not similarly appli- mental factors in fish communities of 146 palustrine cable to different ecoregions. Our results showed that wetlands, covering a wide range of altitude and wetland the habitat diversity, macroinvertebrate richness, altitude surface area in Neotropical region. Two questions were and hydroperiod were the environmental predictors that analyzed: (1) Are wetland altitude, area, habitat diversi- potentially structure and maintain the fish occurrence ty, hydroperiod (permanent and intermittent), ecoregion, and richness in southern Brazil palustrine wetlands. and macroinvertebrate richness good predictors of oc- Such information is essential to develop wetland con- currence, richness, abundance and composition of fish servation and management programs in this region, species? and (2) Are the predictors of fish richness where more than 90 % of wetland systems have already similarly applicable to different ecoregions in Southern been lost and the remaining ones are still at high risk due Brazil? Our data showed that fish richness was to the anthropogenic activities. related to habitat diversity and macroinvertebrate richness, and fish occurrence was influenced by Keywords South American fishes . Ecoregions . wetland area and macroinvertebrate richness. Fish Ichthyofauna . Biodiversity conservation abundance was influenced by altitude, hydroperiod and macroinvertebrate richness, and the fish composition was jointly associated with ecoregion, and hydroperiod. -

Diversidade, Padrões De Distribuição E Conservação Dos Peixes Da Caatinga
3. Peixes da Caatinga 3 DIVERSIDADE, PADRÕES DE DISTRIBUIÇÃO E CONSERVAÇÃO DOS PEIXES DA CAATINGA Ricardo S. Rosa, Naércio A. Menezes, Heraldo A. Britski, Wilson J. E. M. Costa & Fernando Groth Introdução O conjunto de espécies de peixes de água doce que ocorre na Caatinga representa o resultado de processos históricos de especiação vicariante, possivelmente determinados por trans- gressões marinhas (Lundberg et al. 1998), expansões do clima semi-árido (Ab’Sáber 1977) e reordenações nas redes de drenagens (Ab’Sáber 1957), de processos ecológicos que determinaram a adaptação de espécies às condições climáticas e o regime hidrológico da região e, finalmente, de processos antrópicos, como as alterações ambientais e os programas de erradicação e introdução de espécies, que possivelmente levaram à exclusão de elementos autóctones da fauna original. Esta ictiofauna inclui representantes de diferentes grupos neotropicais típicos, mas que com exceção dos peixes anuais (Rivulidae), mostra-se bem menos diversificada quando comparada à de outros ecossistemas brasileiros. Suas espécies distribuem-se em bacias interiores e costeiras do nordeste brasileiro, que drenam parcialmente ou estão inteiramente localizadas na Caatinga. Por isso, não há como caracterizar uma ictiofauna típica ou exclusiva deste ecossistema, já que a distribuição de muitas espécies nos rios que cortam a Caatinga estende-se para além de 135 R.S. Rosa et al. seus limites, atingindo outros ecossistemas adjacentes do nordeste brasileiro e regiões vizinhas. O endemismo da fauna de peixes do nordeste brasileiro foi reconhecido por Vari (1988), que definiu uma região denominada “Northeastern”, e por Menezes (1996), que incluiu os rios do nordeste como parte de “Northeastern Small Drainages”. -

In This Issue
The Journal of the Catfish Study Group (UK) In this issue Leaf Litter By Peter Liptrot Some notes on the natural diet of Megalancistrus parananus by Lee Finley So you want to breed 'Cory's' by lan Fuller Volume 5 Issue Number 1 March 2004 CONTENTS 1 Committee 2 From the Chair lan Fuller 3 Some notes on the natural diet of Megalancistrus parananus by Lee Finley 5 Meet the Members - Eric Bodrock 6 Leaf Litter by Peter Liptrot 9 Breeders List 11 Project Report by Stephen Pritchard 13 Letter from the Membership Secretary 14 So you want to breed 'Gory's' 18 New 'C' numbers introduced 19 Breeding Aspidoras 'gold' by Adrian Taylor 20 Meet Jonathan Armbruster 21 Changing Rooms - The Finishing Touch by Danny Blundell Articles and pictures can be sent by e-mail direct to the editor <bill@ catfish.co.uk> or by post to Bill Hurst 18 Three Pools Crossens SOUTH PORT PR9 8RA (England) ACKNOWLEDGEMENTS Front Cover: Original Design by Kathy Jinkins. March 2004 Vol 5 No 1 HONORARY CO MMITTEE FOR T HE CAffi'IIJSII Sfffi8F C80fl, (ffl•l 2004 PRESIDENT AUCTION ORGANISERS Trevor (JT) Morris Roy & Dave Barton VICE PRESIDENT FUNCTIONS MANAGER Dr Peter Burgess Trevor Morris [email protected] SOCIAL SECRETARY CHAIRMAN Terry Ward lan Fuller ian @corycats.com WEB SITE MANAGER All an James all an@ scotcat.com VICE CHAIRMAN Danny Blundell COMMITTEE MEMBER [email protected] Peter Liptrot bolnathist@ gn .apc.org SECRETARY Temporarily lan Fuller SOUTHERN REP Steve Pritchard TREASURER S .Pritchard@ bti nternet.com Temporarily: Danny Blundell [email protected] -

Long Term Evolutionary Responses to Whole Genome Duplication
Unicentre CH-1015 Lausanne http://serval.unil.ch Year : 2016 Long term evolutionary responses to whole genome duplication Sacha Laurent Sacha Laurent, 2016, Long term evolutionary responses to whole genome duplication Originally published at : Thesis, University of Lausanne Posted at the University of Lausanne Open Archive http://serval.unil.ch Document URN : urn:nbn:ch:serval-BIB_8E5536BAC6042 Droits d’auteur L'Université de Lausanne attire expressément l'attention des utilisateurs sur le fait que tous les documents publiés dans l'Archive SERVAL sont protégés par le droit d'auteur, conformément à la loi fédérale sur le droit d'auteur et les droits voisins (LDA). A ce titre, il est indispensable d'obtenir le consentement préalable de l'auteur et/ou de l’éditeur avant toute utilisation d'une oeuvre ou d'une partie d'une oeuvre ne relevant pas d'une utilisation à des fins personnelles au sens de la LDA (art. 19, al. 1 lettre a). A défaut, tout contrevenant s'expose aux sanctions prévues par cette loi. Nous déclinons toute responsabilité en la matière. Copyright The University of Lausanne expressly draws the attention of users to the fact that all documents published in the SERVAL Archive are protected by copyright in accordance with federal law on copyright and similar rights (LDA). Accordingly it is indispensable to obtain prior consent from the author and/or publisher before any use of a work or part of a work for purposes other than personal use within the meaning of LDA (art. 19, para. 1 letter a). Failure to do so will expose offenders to the sanctions laid down by this law. -
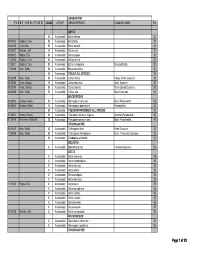
MAS BAP Species Point Table
ANABANTOID F I R S T V E R I F I E D CLASS GROUP GENUS/SPECIES COMMON NAME Pts BETTA B Anabantoid Betta edithae 10 05/01/03 Wojtech, Tom B Anabantoid Betta falax 10 09/24/95 Cram, Dan B Anabantoid Betta imbellis 10 02/06/07 Michels, Jeff B Anabantoid Betta picta 10 09/28/02 Rogne, Eric B Anabantoid Betta pugnax 10 11/25/00 Wojtech, Tom B Anabantoid Betta pulchra 10 11/25/01 Wojtech, Tom B Anabantoid Betta smaragdina Emerald Betta. 10 11/03/88 Kern, Wally B Anabantoid Betta splendens 10 B Anabantoid COLISA ALL SPECIES 10 07/23/89 Kern, Wally B Anabantoid Colisa chuna Honey Dwarf Gourami. 10 02/28/96 Tews, Woody B Anabantoid Colisa fasciatus Giant Gourami. 10 03/13/95 Tews, Woody B Anabantoid Colisa labiosa Thick-Lipped Gourami. 10 06/28/88 Kern, Wally B Anabantoid Colisa lalia Dwarf Gourami. 10 MACROPODUS 09/28/02 Korotev, Kevin B Anabantoid Macropodus concolor Black Paradisefish. 10 04/28/02 Korotev, Kevin B Anabantoid Macropodus opercularis Paradisefish. 10 B Anabantoid PSEUDOSPHROMENUS ALL SPECIES 10 01/25/03 Korotev, Kevin B Anabantoid Pseudosphromenus cupanis Spiketail Paradisefish. 10 01/28/96 Kelnhofer,, Rob & El B Anabantoid Pseudosphromenuspy dayi Day'sy Paradisefish. 10 TRICHOGASTER 05/11/87 Kern, Wally B Anabantoid Trichogaster leeri Pearl Gourami. 10 12/05/88 Kern, Wally B Anabantoid Trichogaster trichopterus Blue, Three-spot, Gourami. 10 B Anabantoid Trichopsis schalleri 10 BELONTIA C Anabantoid Belontia signata Combtail Gourami. 15 BETTA C Anabantoid Betta akarensis 15 C Anabantoid Betta anabantoides 15 C Anabantoid Betta -

Dezembro Nº134
#VacinaJá DE N. 134 - ISSN 1808-1436 SÃO CARLOS, DEZEMBRO/2020 EDITORIAL ueridas associadas e associados, Q Iniciamos aqui a última edição de 2020 do Boletim da SBI. Após um ano desafiador, renovamos nossas esperanças com as notícias promissoras sobre a chegada de vacinas. Primeiramente, gostaríamos de atualizá-los sobre um aspecto bastante importante: as Eleições para a Diretoria e Conselho Deliberativo da nossa sociedade. Conforme comunicamos anteriormente, em função da pandemia do novo coronavírus e do adiamento do EBI 2021, as próximas eleições para os cargos de Diretoria e do Conselho Deliberativo, que ocorrem a cada dois anos, acontecerão de maneira remota em 2021, por meio de plataforma online já contratada. No início deste Editorial explicamos um pouco mais sobre como ocorrerão as eleições. Esperamos que leiam atentamente e nos contatem caso tenham dúvidas. VAGAS A SEREM PREENCHIDAS 1) Diretoria: são 3 (três) vagas para as funções de Presidente, Secretário(a) e Tesoureiro(a). 2) Conselho Deliberativo da SBI: o Conselho Deliberativo é composto por 7 (sete) membros. Duas destas vagas se encontram preenchidas por membros eleitos em 2019 para gestões de 4 (quatro) anos, e uma terceira vaga será automaticamente ocupada pela atual Presidente da SBI, assim que encerrar sua gestão. Portanto, em 2021 serão preenchidas 4 vagas para o Conselho Deliberativo, uma delas para gestão de 4 (quatro) anos (o/a candidato/a mais votado/a) e outras 3 vagas para gestões de 2 (dois) anos. 2 EDITORIAL Chapas inscritas para a Diretoria e candidatos ao Conselho Deliberativo da SBI As inscrições foram realizadas nas duas primeiras semanas de novembro, entre os dias 1 e 14/11/2020. -

Gabinete Do Ministro Instrução Normativa
Diário Oficial da União – Seção I, Nº3, quarta-feira, 4 de janeiro de 2012, páginas 26 a 42 – ISSN 1677-7042 GABINETE DO MINISTRO INSTRUÇÃO NORMATIVA INTERMINISTERIAL N°1, DE 3 DE JANEIRO DE 2012 Estabelece normas, critérios e padrões para a explotação de peixes nativos ou exóticos de águas continentais com finalidade ornamental ou de aquariofilia. O MINISTRO DE ESTADO DA PESCA E AQUICULTURA e a MINISTRA DE ESTADO DO MEIO AMBIENTE no uso de suas atribuições, e tendo em vista o disposto nas Leis n°s 10.683, de 28 de maio de 2003, e 11.959, de 29 de junho de 2009, bem como o constante do Processo IBAMA/Sede nº 02001.002681/04- 06, resolvem: Art.1º - Estabelecer normas, critérios e padrões para a explotação de peixes nativos ou exóticos de águas continentais com finalidade ornamental ou de aquariofilia. Parágrafo único. Esta Instrução Normativa Interministerial não se aplica às seguintes situações: I - exposição em restaurantes, para fins de consumo alimentar de peixes vivos; e II - exposição de peixes vivos em zoológicos, mostras ou similares com finalidade didática, educacional ou científica. CAPÍTULO I DAS DISPOSIÇÕES PRELIMINARES Art. 2º - Para efeito desta Instrução Normativa Interministerial, considera-se: I - Ornamentação: utilizar organismos vivos ou não, para fins decorativos, ilustrativos ou de lazer; e II - Aquariofilia: manter ou comercializar, para fins de lazer ou de entretenimento, indivíduos vivos em aquários, tanques, lagos ou reservatórios de qualquer tipo. CAPÍTULO II DA CAPTURA E EXPLOTAÇÃO Art. 3º - Fica permitida a captura, o transporte e a comercialização de exemplares vivos de peixes nativos das espécies listadas no Anexo I desta Instrução Normativa Interministerial. -

Vinicius Renner Lampert Análise De Atributos
VINICIUS RENNER LAMPERT ANÁLISE DE ATRIBUTOS ECOLÓGICOS DA ICTIOFAUNA EM UMA DRENAGEM COSTEIRA NO SUL DO BRASIL Tese apresentada ao Programa de Pós-Graduação em Biologia Animal, Instituto de Biociências, Universidade Federal do Rio Grande do Sul, como requisito parcial à obtenção do título de Doutor em Biologia Animal. Área de Concentração: Biodiversidade Orientador: Profa. Dra. Clarice Bernhardt Fialho UNIVERSIDADE FEDERAL DO RIO GRANDE DO SUL PORTO ALEGRE 2013 ANÁLISE DE ATRIBUTOS ECOLÓGICOS DA ICTIOFAUNA EM UMA DRENAGEM COSTEIRA NO SUL DO BRASIL VINICIUS RENNER LAMPERT Aprovada em ______ de ______________ de 2013. ____________________________ Prof. Dr. Ana Paula Sassanovicz Dufech ____________________________ Profa. Dra. Carla Ferreira Rezende ____________________________ Prof. Dr. Yzel Rondon Súarez ____________________________ Profa. Dra. Clarice Bernhardt Fialho ii “Dream on, dream on Dream until your dream comes true”. (Steven Tyler - Aerosmith) iii AGRADECIMENTOS Talvez este espaço seja um dos mais importantes desta tese, não em termos de conteúdo, mas em função de que ele é dedicado ao reconhecimento àqueles que serviram de referência, inspiração e exemplo para guiar minha vida tanto pessoal, como acadêmica/profissional, desde muito antes de entrar para a faculdade, ajudando a moldar o que sou hoje. Um evento e um amigo de infância, muito provavelmente foram determinantes para me direcionar, muitos anos mais tarde, ao estudo dos peixes. Esse evento foi o fato de eu ganhar um aquário do meu pai, por influência desse amigo. Por isso gostaria de dedicar meu trabalho até hoje a eles: meu pai, Fernão Mottin Lampert pelo empurrão inicial; e meu amigo Paulo Marcos Führ, que desde o início me ajudou a descobrir e conhecer o mundo dos peixes. -

Corydorasworld
Volume 15, Issue 1. February 2014 The Journal of The CaTfish sTudy Group Furthering the study of catfish PlanetXingu Update Focus on Scleromystax Yorkshire Aquarist Club Tour CSG Autumn Auction An Introduction to Hypancistrus What’s New? The Latest CW Numbers Hot Cory’s Seeing the Light Volume 15, Issue 1. February 2014 Catfish Study Group Committee President – Ian Fuller Secretary – Ian Fuller [email protected] [email protected] [email protected] Editor – Mark Walters Vice-President – Dr. Peter Burgess [email protected] Chairman – Bob Barnes BAP Secretary – Brian Walsh [email protected] [email protected] Treasurer – Danny Blundell Show Secretary – Brian Walsh [email protected] [email protected] Membership Secretary – Mike O’Sullivan Assistant Show Secretary – Ann Blundell [email protected] Website Manager – Allan James Auction Manager – David Barton [email protected] [email protected] Floor Member – Bill Hurst Assistant Auction Manager – Roy Barton [email protected] Scientific Advisor – Dr. Michael Hardman Diary Dates - 2014 Date Meeting Venue February 16th Spring Auction Derwent Hall March 14th/15th/16th Annual Convention Kilhey Court Hotel April 27th Discussion on filtration Darwen Valley Community Centre May 18th Discussion on Catfish breeding Darwen Valley Community Centre June 8th Summer lectures and Sales Meet Derwent Hall July and August