Investigating the Feasibility to Remove Shp Pandeiros: Lessons from Fish Fauna
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Long Term Evolutionary Responses to Whole Genome Duplication
Unicentre CH-1015 Lausanne http://serval.unil.ch Year : 2016 Long term evolutionary responses to whole genome duplication Sacha Laurent Sacha Laurent, 2016, Long term evolutionary responses to whole genome duplication Originally published at : Thesis, University of Lausanne Posted at the University of Lausanne Open Archive http://serval.unil.ch Document URN : urn:nbn:ch:serval-BIB_8E5536BAC6042 Droits d’auteur L'Université de Lausanne attire expressément l'attention des utilisateurs sur le fait que tous les documents publiés dans l'Archive SERVAL sont protégés par le droit d'auteur, conformément à la loi fédérale sur le droit d'auteur et les droits voisins (LDA). A ce titre, il est indispensable d'obtenir le consentement préalable de l'auteur et/ou de l’éditeur avant toute utilisation d'une oeuvre ou d'une partie d'une oeuvre ne relevant pas d'une utilisation à des fins personnelles au sens de la LDA (art. 19, al. 1 lettre a). A défaut, tout contrevenant s'expose aux sanctions prévues par cette loi. Nous déclinons toute responsabilité en la matière. Copyright The University of Lausanne expressly draws the attention of users to the fact that all documents published in the SERVAL Archive are protected by copyright in accordance with federal law on copyright and similar rights (LDA). Accordingly it is indispensable to obtain prior consent from the author and/or publisher before any use of a work or part of a work for purposes other than personal use within the meaning of LDA (art. 19, para. 1 letter a). Failure to do so will expose offenders to the sanctions laid down by this law. -
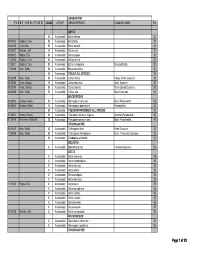
MAS BAP Species Point Table
ANABANTOID F I R S T V E R I F I E D CLASS GROUP GENUS/SPECIES COMMON NAME Pts BETTA B Anabantoid Betta edithae 10 05/01/03 Wojtech, Tom B Anabantoid Betta falax 10 09/24/95 Cram, Dan B Anabantoid Betta imbellis 10 02/06/07 Michels, Jeff B Anabantoid Betta picta 10 09/28/02 Rogne, Eric B Anabantoid Betta pugnax 10 11/25/00 Wojtech, Tom B Anabantoid Betta pulchra 10 11/25/01 Wojtech, Tom B Anabantoid Betta smaragdina Emerald Betta. 10 11/03/88 Kern, Wally B Anabantoid Betta splendens 10 B Anabantoid COLISA ALL SPECIES 10 07/23/89 Kern, Wally B Anabantoid Colisa chuna Honey Dwarf Gourami. 10 02/28/96 Tews, Woody B Anabantoid Colisa fasciatus Giant Gourami. 10 03/13/95 Tews, Woody B Anabantoid Colisa labiosa Thick-Lipped Gourami. 10 06/28/88 Kern, Wally B Anabantoid Colisa lalia Dwarf Gourami. 10 MACROPODUS 09/28/02 Korotev, Kevin B Anabantoid Macropodus concolor Black Paradisefish. 10 04/28/02 Korotev, Kevin B Anabantoid Macropodus opercularis Paradisefish. 10 B Anabantoid PSEUDOSPHROMENUS ALL SPECIES 10 01/25/03 Korotev, Kevin B Anabantoid Pseudosphromenus cupanis Spiketail Paradisefish. 10 01/28/96 Kelnhofer,, Rob & El B Anabantoid Pseudosphromenuspy dayi Day'sy Paradisefish. 10 TRICHOGASTER 05/11/87 Kern, Wally B Anabantoid Trichogaster leeri Pearl Gourami. 10 12/05/88 Kern, Wally B Anabantoid Trichogaster trichopterus Blue, Three-spot, Gourami. 10 B Anabantoid Trichopsis schalleri 10 BELONTIA C Anabantoid Belontia signata Combtail Gourami. 15 BETTA C Anabantoid Betta akarensis 15 C Anabantoid Betta anabantoides 15 C Anabantoid Betta -
Corydoras Diphyes (Siluriformes: Callichthyidae) and Otocinclus Mimulus (Siluriformes: Loricariidae), Two New Species of Catfishes from Paraguay, a Case of …
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/228490534 Corydoras diphyes (Siluriformes: Callichthyidae) and Otocinclus mimulus (Siluriformes: Loricariidae), two new species of catfishes from Paraguay, a case of … Article in Ichthyological Exploration of Freshwaters · November 2003 CITATIONS READS 24 351 2 authors: Thomas Axenrot Sven Kullander Swedish University of Agricultural Sciences Swedish Museum of Natural History 16 PUBLICATIONS 259 CITATIONS 195 PUBLICATIONS 1,679 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: A Pan-European Species-directories Infrastructure (PESI) View project Stock and ecosystem analysis View project All content following this page was uploaded by Sven Kullander on 22 October 2014. The user has requested enhancement of the downloaded file. 249 Ichthyol. Explor. Freshwaters, Vol. 14, No. 3, pp. 249-272, 7 figs., 11 tabs., October 2003 © 2003 by Verlag Dr. Friedrich Pfeil, München, Germany – ISSN 0936-9902 Corydoras diphyes (Siluriformes: Callichthyidae) and Otocinclus mimulus (Siluriformes: Loricariidae), two new species of catfishes from Paraguay, a case of mimetic association Thomas E. Axenrot* and Sven O. Kullander** Corydoras diphyes and O. mimulus, new species, are found in association in tributaries to the Río Monday, a right bank tributary of the Río Paraná, and are conspicuously similar to each other in color pattern. Otocinclus mimulus has been confused with O. flexilis up till now. Otocinclus mimulus mimics O. diphyes. The mimesis is unusual because the two species occupy different microhabitats and it is hypothesized to operate with a primarily visual predator moving between the microhabitats, tentatively identified as the cichlid Crenicichla lepidota. -

Boletim De Pesquisa PEIXES ONÍVOROS DA PLANÍCIE
Boletim de Pesquisa ISSN 1517-1981 Número, 16 Outubro, 2000 PEIXES ONÍVOROS DA PLANÍCIE INUNDÁVEL DO RIO MIRANDA, PANTANAL, MATO GROSSO DO SUL, BRASIL Boletim de Pesquisa Nº 16 ISSN 1517-1981 PEIXES ONÍVOROS DA PLANÍCIE INUNDÁVEL DO RIO MIRANDA, PANTANAL, MATO GROSSO DO SUL, BRASIL Emiko Kawakami de Resende Rosana Aparecida Cândido Pereira Vera Lúcia Lescano de Almeida Ana Geise da Silva Corumbá 2000 Embrapa Pantanal. Boletim de Pesquisa, 16 Exemplares desta publicação podem ser solicitados à Embrapa Pantanal Rua 21 de Setembro, 1880 Caixa Postal 109 Telefone: (67) 233-2430 Fax: (67) 233-1011 E-mail: [email protected] 79320-900 Corumbá, MS Homepage: www.cpap.embrapa.br Comitê de Publicações: Emiko Kawakami de Resende - Presidente Vânia da Silva Nunes - Secretária Executivo Balbina Maria Araújo Soriano Cristina Aparecida Gonçalves Rodrigues André Steffens Moraes Regina Célia Rachel dos Santos - Secretária 1ª edição: 1ª impressão (2000): 200 exemplares 2ª edição (2002): Formato digital RESENDE, E.K. de; PEREIRA, R.A.C. Peixes onívoros da planície inundável do rio Miranda, Pantanal, Mato Grosso do Sul, Brasil. Corumbá: Embrapa Pantanal, 2000. 44p. (Embrapa Pantanal. Boletim de Pesquisa,16). ISSN 1517-1981 1. Peixe - Comunidade - Alimentação. 2. Peixe onívoro - Pantanal. 3. Pantanal – Peixe onívoro - Rio Miranda - Mato Grosso do Sul. I. Embrapa Pantanal (Corumbá, MS). II. Título. III. Série. CDD: 597.098171 Copyright Embrapa-2000 SUMÁRIO Pág. RESUMO ............................................................................................. -

A Biological Assessment of the Aquatic Ecosystems of the Pantanal, Mato Grosso Do Sul, Brasil
Rapid Assessment Program Programa de Avaliação Rápida 18 RAP Bulletin of Biological Assessment RAP Buletim de Avaliação Biológica A Biological Assessment of the Aquatic Ecosystems of the Pantanal, Mato Grosso do Sul, Brasil Uma avaliação biológica dos ecossistemas aquáticos do Pantanal, Mato Grosso do Sul, Brasil CENTER FOR APPLIED BIODIVERSITY SCIENCE Philip W. Willink, Barry Chernoff, Leeanne E. Alonso, (CABS) CONSERVATION INTERNATIONAL Jensen R. Montambault, and Reinaldo Lourival, THE FIELD MUSEUM Editors, Editores MUSEU DE ZOOLOGIA, UNIVERSIDADE DE SÃO PAULO EMBRAPA UNIVERSIDADE FEDERAL DE MATO GROSSO DO SUL CONSERVATION PRIORITIES: THE ROLE OF RAP Our planet faces many serious environmental problems, among them global climate change, soil erosion, and pollution. At Conservation International (CI), we believe that there is one problem that surpasses all others in terms of importance because of its irreversibility, the extinction of biological diversity. Conservation efforts still receive only a tiny fraction of the resources, both human and financial, needed to get the job done. As a result of this, we must use available resources efficiently, applying them to those places with the highest concentrations of diversity that are at most immediate risk of disappearing. CI uses a strategic approach for setting conservation investment priorities. At a global level, we have targeted the “hotspots”, twenty-five areas that hold a third or more of all terrestrial diversity and are at a great risk. Our global priorities also focus on major tropical wilderness areas and the “mega-diversity” country concept, which highlights the importance of the national entities that harbor high biodiversity. We are now undertaking a series of priority-setting exercises for other major categories of ecosystems, among them marine systems, freshwater aquatic systems, deserts, and dry forests. -

Splash-August-September-2001
THE SPLASH THE OFFICIAL PUBLICATION OF THE MILWAUKEE AQUARIUM SOCIETY, INC. Photo courtesy of Dwight Lehman In this Issue: Betta albimarginata Plus Little Yellow Catfish With Spots?? August General Meeting – Jim Gasior Killifish August/September 2001 MILWAUKEE AQUARIUM SOCIETY SOCIETY OFFICERS President: Dwight Lehman (414) 332-3735 Vice President: Ken King (262) 284-2684 Secretary: Heather Hertziger (414) 875-7831 Treasurer: Jerry Michels (414) 353-5370 Sgt. At Arms: Virginia Lehman (414) 332-3735 BOARD OF DIRECTORS Chairperson: Ray Gettler (262) 662-5591 Splash Editor: Naomi Gettler (262) 662-5591 Board Members: Bonnie King (262) 284-2684 Joe Martin (262) 252-3148 Ron Revolinski (414) 353-7680 Bob Suchocki (262) 246-8739 Past President: Ray Gettler (262) 662-5591 THE SPLASH STAFF Editor: Naomi Gettler (262) 662-5591 Exchange Editor: Ralph Bahrke (262) 377-6546 Technical Editor Joe Martin (262) 252-3148 Publisher: Judy Martin (262) 252-3148 COMMITTEE CHAIRPERSONS Breeders Award Program (BAP): Kevin Korotev (414) 332-9785 Tom Wojtech (414) 527-0399 Librarian: Bob Suchocki (262) 246-8739 Participation Awards (PAP): Bonnie King (262) 284-2684 Program Committee: Dwight Lehman (414) 332-3735 Ray Gettler (262) 662-5591 Membership: Judy Martin (262) 252-3148 Bowl Show: Ken King (262) 284-2684 VISIT US AT OUR WEB SITE AT: www.fishclubs.com/WI/MAS PRESIDENT’S PAGE From the desk of the President: Here it is August already. The summer is moving right along. There are still many club activities to look forward to between now and the end of the year. We have Jim Gasior talking about killies at our August meeting and Charlie Grimes talking about diving in Mexico at our September meeting. -

Tank Topics July/August 2015
The Greater Akron Aquarium Society Tank Topics July/August 2015 A red & white Ryukin goldfish, one of the Inside this issue: divisional winners from this year’s show. President’s Message 3 Bud White See all the results Editor’s Message 3 starting on page 7 Dave Williamson BAP/HAP 4 Wayne Toven Bowl Show 5 Don Youngkin Exchange Review 6 Wayne Toven Ultra-Aqua 2015 7 Show Results Coming Events 10 Meeting Notice 10 2015 GAAS Board of Directors President ....................... Bud White .............................. (330) 571-0394/[email protected] Vice President ............... Jeff Plazak .............................. (330) 854-5257/[email protected] Treasurer ...................... Rich Serva ............................. (330) 650-4613/[email protected] Secretary....................... Dave Girard ................................................. [email protected] Important Dates Editor ............................. Dave Williamson .......................................... [email protected] for 2015 Special Activities ........... Don Youngkin ........................................... [email protected] BAP/HAP ...................... Wayne Toven ..................... (330) 256-7836/[email protected] March 1 Membership .................. Bill Schake .......................................................... [email protected] Spring auction Raffle ............................. Phil & Tiffany Hypes ............... (330) 327-6316/[email protected] Historian ........................ Steve Brunn ......................................... -
Corydoras Diphyes (Siluriformes: Callichthyidae) and Otocinclus
249 Ichthyol. Explor. Freshwaters, Vol. 14, No. 3, pp. 249-272, 7 figs., 11 tabs., October 2003 © 2003 by Verlag Dr. Friedrich Pfeil, München, Germany – ISSN 0936-9902 Corydoras diphyes (Siluriformes: Callichthyidae) and Otocinclus mimulus (Siluriformes: Loricariidae), two new species of catfishes from Paraguay, a case of mimetic association Thomas E. Axenrot* and Sven O. Kullander** Corydoras diphyes and O. mimulus, new species, are found in association in tributaries to the Río Monday, a right bank tributary of the Río Paraná, and are conspicuously similar to each other in color pattern. Otocinclus mimulus has been confused with O. flexilis up till now. Otocinclus mimulus mimics O. diphyes. The mimesis is unusual because the two species occupy different microhabitats and it is hypothesized to operate with a primarily visual predator moving between the microhabitats, tentatively identified as the cichlid Crenicichla lepidota. The mimetic association expressed by C. diphyes and O. mimulus extends to other species pairs of the same genera in southern South America with similarly impressive agreement in coloration, but the operating mechanism is not known in these pairs. In a cladistic analysis of Otocinclus using morphological characters, O. mimulus is included in a clade consisting also of O. flexilis and O. affinis. Addition of mimetic similarity results in a single most parsimonious tree with O. mimulus, O. flexilis, O. affinis and O. xakriaba forming a monophyletic group. Introduction purpose of this paper is to describe the new taxa and to discuss their mimetic association. In 1998, one of us (SOK) sampled several locali- Otocinclus is a genus of small hypoptopoma- ties in the vicinity of Ciudad del Este and ob- tine loricariid catfishes living in groups or schools. -
Corydoras Desana, a New Plated Catfish from the Upper Rio Negro, Brazil, with Comments on Mimicry Within Corydoradinae (Ostariophysi: Siluriformes: Callichthyidae)
aqua, International Journal of Ichthyology Corydoras desana, a new plated catfish from the upper rio Negro, Brazil, with comments on mimicry within Corydoradinae (Ostariophysi: Siluriformes: Callichthyidae) Flávio C. T. Lima1, 2 and Ivan Sazima1 1) Museu de Zoologia da Universidade Estadual de Campinas “Adão José Cardoso”, Caixa Postal 6109, 13083-863, Campinas, SP, Brazil. 2) Corresponding author. E-mail: [email protected] Accepted: 11 January 2017 Keywords mente mais abundante. Com base na morfologia do espin- Corydoras tukano, Rio Tiquié, Rio Uaupés, Lineage 1, ho peitoral, é aqui sugerido que as espécies de Corydoras Batesian mimicry. que pertencem à linhagem 1 são mímicos Batesianos de congêneres pertencentes às linhagens 6 a 9, ao invés de co- Abstract mímicos Müllerianos como recentemente suposto. A new Corydoras species is described from the Igarapé Castanha, a tributary of rio Tiquié, upper rio Negro basin, Résumé Brazil. The new species belongs to a basal clade of long- Une nouvelle espèce de Corydoras est décrite originaire de snouted Corydoras (Lineage 1 or Corydoras acutus species- l’Igarapé Castanha, un tributaire du rio Tiquié, bassin du group) and occurs syntopically with a co-mimic species, haut rio Negro, Brésil. La nouvelle espèce fait partie d’un Corydoras tukano, which is considerably more abundant. clade de base de Corydoras à long rostre (Lignage 1 ou Based on the pectoral-spine morphology, it is herein sug- groupe d’espèces Corydoras acutus) et vit syntopiquement gested that Corydoras species belonging to the Lineage 1 are avec une espèce co-mimétique, Corydoras tukano, qui est Batesian mimics of congeners belonging to Lineages 6 to 9, considérablement plus abondant. -
Analysis of the Ornamental Fish Exports from the Amazon State, Brazil*
BOLETIM DO INSTITUTO DE PESCA ISSN 1678-2305 online version Scientific Article ANALYSIS OF THE ORNAMENTAL FISH EXPORTS FROM THE AMAZON STATE, BRAZIL* ABSTRACT The updated status of the ornamental fish trade from the Amazonas state was analyzed between Ivan de Azevedo TRIBUZY-NETO1 the years of 2006-2015 (IBAMA database). The trade of ornamental fish from Amazonas State mainly consists of wild species. A total of 142,552,253 specimens were exported during the studied Hélio BELTRÃO2* period. Sales plummeted since 2006 from 26,075,241 specimens exported to 2,729,846 specimens Zehev Schwartz BENZAKEN3 in 2015 (Jan-Jul). Between 2006 and 2015, a total of US$ 23.0 million in revenue was generated from fish exports destined to 35 countries. Germany, Taiwan, USA and Japan accounted for 75.5% of Kedma Cristine YAMAMOTO2 the volume and 76.7% of the value exported. During this time 375 species were exported, included Paracheirodon axelrodi, P. simulans, Hemigrammus bleheri, Otociclus affinis and O. hoppei that together represented 84.5% of exports. Thirty of them are not on the list of Brazilian Institute of the Environment and Renewable Natural Resources - IBAMA species released for export, and six are currently on the list of endangered fauna of Brazil: Hopliancistrus tricornis, Leporacanthicus joselimai, Parancistrus nudiventris, Peckoltia compta, Scobinancistrus auratus and S. pariolispos. 1 Universidade Federal do Amazonas – UFAM, Laboratório The collection of this information can help producers, managers and environmentalists in the de Ictiologia – LABIC. Av. General Rodrigo Otávio, elaboration of the political policies to establish regulations to govern the trade. 3.000, CEP: 69077-000, Manaus, AM, Brazil. -
Reconstitution of the Reophylic Ichthyofauna of the Lower São Francisco
INTEGRATED MANAGEMENT OF LAND BASED ACTIVITIES IN THE SAN FRANCISCO RIVER BASIN PROJECT GEF/ANA/OAS/UNEP Activity 1.3 – Reophylic Ichthyofauna Recuperation in the Lower São Francisco River Basin Executive Summary of the Final Report RECONSTITUTION OF THE REOPHYLIC ICHTHYOFAUNA OF THE LOWER SÃO FRANCISCO Instituto de Desenvolvimento Científico e Tecnológico de Xingó Canindé do São Francisco - SE INTEGRATED MANAGEMENT OF LAND BASED ACTIVITIES IN THE SAN FRANCISCO RIVER BASIN PROJECT GEF/ANA/OAS/UNEP Activity 1.3 – Reophylic Ichthyofauna Recuperation in the Lower São Francisco River Basin Executive Summary of the Final Report RECONSTITUTION OF THE REOPHYLIC ICHTHYOFAUNA OF THE LOWER SÃO FRANCISCO Coordination Fábio José Castelo Branco Costa Instituto de Desenvolvimento Cientifico e Tecnológico de Xingó Participants Enaide Marinho de Melo Magalhães LABMAR/UFAL Maria Célia de Andrade Lyra LABMAR/UFAL Manoel Messias dos Santos LABMAR/UFAL Rivaldo Couto dos Santos Júnior Instituto Xingó Sineide Correia Silva Montenegro UFAL April2003 RECONSTITUTION OF THE REOPHYLIC ICHTHYOFAUNA OF THE LOWER SÃO FRANCISCO Executive Summary INTRODUCTION Activity 1.3, “Reophylic Ichthyofauna Recuperation in the Lower São Francisco Basin”, is part of Component I (Environmental Assessment of the Basin) of the Integrated Management of Land Based Activities in the San Francisco River Basin Project, whose main objectives are the identification and assessment of the degree to which inland activities and the regulation of the River affect the hydrology, the water quality (particularly sediment and nutrient transportation), fishing and the aquatic environment in the Basin and in the adjacent coastal zone. Among the objectives there are: 1)Surveying fish species and limnologic characterization of the Xingó Reservoir and of the downstream stretch, up to the Ocean. -

Zootaxa, Checklist of Catfishes, Recent and Fossil
ZOOTAXA 1418 Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types CARL J. FERRARIS, JR. Magnolia Press Auckland, New Zealand CARL J. FERRARIS, JR. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types (Zootaxa 1418) 628 pp.; 30 cm. 8 March 2007 ISBN 978-1-86977-058-7 (hardback) ISBN 978-1-86977-059-4 (Online edition) FIRST PUBLISHED IN 2007 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2007 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 1418 © 2007 Magnolia Press FERRARIS Zootaxa 1418: 1–628 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types CARL J. FERRARIS, JR. 2944 NE Couch Street, Portland, Oregon, 97232, U.S.A. E-mail: [email protected] Research Associate, National Museum of Natural History, Smithsonian Institution, Washington Research Associate, American Museum of Natural History, New York Research Associate and Honorary Fellow, California Academy of Sciences, San Francisco Table of contents Abstract ....................................................................................................................................................................................................