Preliminary Analysis of Beak Stable Isotopes (Δ13c and Δ15n) Stock Variation of Neon Flying Squid, Ommastrephes Bartramii, in the North Pacific Ocean
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Length-Weight Relationship of Neon Flying Squid Ommastrephes Bartramii (Cephalopoda: Ommastrephidae) Caught from Indian Sector of Southern Ocean
Indian Journal of Geo-Marine Science Vol. 43(8), August 2014, pp 1581-1584 Length-weight relationship of neon flying squid Ommastrephes bartramii (Cephalopoda: Ommastrephidae) caught from Indian sector of Southern Ocean. *Aneesh Kumar K. V1#., Pravin P1., Ragesh N2 & Meenakumari B3. 1Central Institute of Fisheries Technology, Matsyapuri, Willingdon Island. Cochin-682029, India, 2Central Marine Fisheries Research Institute, Cochin- 682018, India, 3Indian Council of Agricultural Research, Krishi Anusandhan Bhavan 2, New Delhi-110012, India #Present address: Centre for Marine Living Resources and Ecology Kendriya Bhavan, CSEZ P.O., Cochin-682037, India *[E. Mail: [email protected]] Received 1 July 2013; revised 7 August 2013 Length-weight relationship of the Neon flying squid Ommastrephes bartramii, caught from the Indian Sector of Southern Ocean was estimated as male W= 0.0235 L 3.05 (R2 = 0.990719) and females W= 0.0283 L 2.99 (R2 = 0.919944). The species follows an isometric growth pattern and no significant difference was observed between both sexes. [Key words: Length- Weight Relation, Squid, Ommastrephes bartramii, Southern Ocean] Introduction Ommastrephes bartramii (Lesueur, 1821) is a morphometric characters gives a better idea for widely distributed oceanic ommastrephid species understanding the relationship between the species throughout the subtropical and temperate waters of and to compare same species in different both northern and southern hemisphere and geographical areas8. The study of the individual excluded from the equatorial waters of all three growth pattern gives an insight about the population oceans1 and forms a major fishery in the Japanese dynamics of the species such as growth and squid fisheries in the Pacific Ocean2. -

Os Nomes Galegos Dos Moluscos
A Chave Os nomes galegos dos moluscos 2017 Citación recomendada / Recommended citation: A Chave (2017): Nomes galegos dos moluscos recomendados pola Chave. http://www.achave.gal/wp-content/uploads/achave_osnomesgalegosdos_moluscos.pdf 1 Notas introdutorias O que contén este documento Neste documento fornécense denominacións para as especies de moluscos galegos (e) ou europeos, e tamén para algunhas das especies exóticas máis coñecidas (xeralmente no ámbito divulgativo, por causa do seu interese científico ou económico, ou por seren moi comúns noutras áreas xeográficas). En total, achéganse nomes galegos para 534 especies de moluscos. A estrutura En primeiro lugar preséntase unha clasificación taxonómica que considera as clases, ordes, superfamilias e familias de moluscos. Aquí apúntase, de maneira xeral, os nomes dos moluscos que hai en cada familia. A seguir vén o corpo do documento, onde se indica, especie por especie, alén do nome científico, os nomes galegos e ingleses de cada molusco (nalgún caso, tamén, o nome xenérico para un grupo deles). Ao final inclúese unha listaxe de referencias bibliográficas que foron utilizadas para a elaboración do presente documento. Nalgunhas desas referencias recolléronse ou propuxéronse nomes galegos para os moluscos, quer xenéricos quer específicos. Outras referencias achegan nomes para os moluscos noutras linguas, que tamén foron tidos en conta. Alén diso, inclúense algunhas fontes básicas a respecto da metodoloxía e dos criterios terminolóxicos empregados. 2 Tratamento terminolóxico De modo moi resumido, traballouse nas seguintes liñas e cos seguintes criterios: En primeiro lugar, aprofundouse no acervo lingüístico galego. A respecto dos nomes dos moluscos, a lingua galega é riquísima e dispomos dunha chea de nomes, tanto específicos (que designan un único animal) como xenéricos (que designan varios animais parecidos). -

Squid and Octopus
Sector Improvement Profile: Squid and Octopus More than half (52%) of global production is yellow- or red-rated, indicating that improvements are needed, and 45% is status unknown. Our improvement efforts prioritize: • The 27% of global squid and octopus production that is red-rated; and • The 39% within the scope of Sustainable Fisheries Partnership’s Target 75 Initiative. Red-rated Species Source country Argentine shortfin squid Argentina Common octopus Mexico, Portugal, Spain Common squids nei Indonesia, Thailand Japanese flying squid Japan Mexican four-eyed octopus Mexico Octopuses nei Indonesia, Mauritania, Morocco, Philippines Various squids nei China, India The Monterey Bay Aquarium’s Seafood Watch Program ratings are based on specific location and production method information and make exceptions for specific brands. For detailed information, visit the Seafood Watch recommendations on squid and octopus. FIP or AIP Where available, the links below lead to detailed information about FIPs listed on FisheryProgress.org. Species Source country Day Octopus Madagascar California Two-Spot Octopus Mexico Giant Pacific Octopus Japan Japanese Flying Squid China Jumbo Squid Mexico, Peru Mitre Squid China Red octopus Chile, Mexico Octopus China The Seafood Certification & Ratings Collaboration unites five global seafood certification and ratings programs working together to coordinate our tools and increase our impact so that more seafood producers move along a clear path toward environmental sustainability and social responsibility. Learn -

During 1979-1997
ICES CM/1988M:39 (Poster) 38.13 4 Interannual Variability in the Neon Flying Squid Abundance and Oceanographic Conditions in the Central North Pacific Ocean during 1979-1997 A. Yatsu*, J. Mori*, T. Watanabe*, T. Meguro", Y. Kamei", and Y. Sakurai" Abstract The neon flying squid, Ommastrephes bartrami, was the target species of the Japanese squid driftnet fishery in the Central North Pacific Ocean during 1979- 1992. Interannual variation in the neon flying squid catch-per-unit-effort (CPUE) in this fishery was highly correlated with that of the Hokkaido University's research driftnet surveys since 1979 along 175' 307Ein July which coincided with the peak of the commercial fishery. While productivity in the northern North Pacific Ocean was high during the 1970's and declining to the present days, the research net CPUE of 0. bartrami was higher in 1979 and in 1994-97 than in other years, suggesting effect of fishing on the stock abundance. The distributions and CPUE variability in 0. bartarmi were also affected by water temperature and salinity structures around the Subarctic Boundary. During the intensive driftnet fishing, three squid species (0. bartrami, Gonatopsis borealis and Onchoteuthis borealijaponica) may have, to some extent, filled throphic niche that was occupied by pelagic fishes. National Research Institute of Far Sear Fisheries, Shimizu 424-8633, Japan " Faculty of Fisheries, Hokknido University, Hakodate 041-8611, Japan Introduction end of 1992 according to the bycatch problem. Since 1993, fishing mortality The neon flying squid, Ommastrephes of the autumn cohort has been derived bartrami, is one of the most dominant only by jig fishing whose annual catch nekton in the epipelagic subtropical and has been less than approximately 10,000 subpolar waters of the world oceans, and tons for this cohort. -

Cephalopoda: Ommastrephidae) in the Southeastern Pacific Revista De Biología Marina Y Oceanografía, Vol
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Nigmatullin, Chingis M.; Shchetinnikov, Alexander S.; Shukhgalter, Olga A. On feeding and helminth fauna of neon flying squid Ommastrephes bartramii (Lesueur, 1821) (Cephalopoda: Ommastrephidae) in the southeastern Pacific Revista de Biología Marina y Oceanografía, vol. 44, núm. 1, abril, 2009, pp. 227-235 Universidad de Valparaíso Viña del Mar, Chile Available in: http://www.redalyc.org/articulo.oa?id=47911450023 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista de Biología Marina y Oceanografía 44(1): 227-235, abril de 2009 On feeding and helminth fauna of neon flying squid Ommastrephes bartramii (Lesueur, 1821) (Cephalopoda: Ommastrephidae) in the southeastern Pacific Alimentación y fauna de helmintos del calamar rojo Ommastrephes bartramii (Cephalopoda: Ommastrephidae) en el Pacífico sudeste Chingis M. Nigmatullin1, Alexander S. Shchetinnikov1 and Olga A. Shukhgalter1 1Atlantic Research Institute of Marine Fisheries and Oceanography (AtlantNIRO), Donskoj Str. 5, Kaliningrad, 236000 Russia [email protected] Resumen.- Se analizó el contenido estomacal de 60 en el 43,3% de los estómagos e incluyó copépodos, ostrácodos, calamares Ommastrephes bartramii (160-392 mm mantle anfípodos, eufáusidos, camarones, moluscos tecosomados, length, ML) recolectados en el Pacífico sudeste (entre 17° y heterópodos y quetognatos. Se encontraron seis especies de 43°S), entre 1981 y 1984. Adicionalmente otros 22 calamares helmintos parásitos en estado larval, con una prevalencia total (165-365 mm ML) fueron examinaron por parásitos helmintos. -

Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries
Advances in Cephalopod Science:Biology, Ecology, Cultivation and Fisheries,Vol 67 (2014) Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book Advances in Marine Biology, Vol. 67 published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: Paul G.K. Rodhouse, Graham J. Pierce, Owen C. Nichols, Warwick H.H. Sauer, Alexander I. Arkhipkin, Vladimir V. Laptikhovsky, Marek R. Lipiński, Jorge E. Ramos, Michaël Gras, Hideaki Kidokoro, Kazuhiro Sadayasu, João Pereira, Evgenia Lefkaditou, Cristina Pita, Maria Gasalla, Manuel Haimovici, Mitsuo Sakai and Nicola Downey. Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries. In Erica A.G. Vidal, editor: Advances in Marine Biology, Vol. 67, Oxford: United Kingdom, 2014, pp. 99-233. ISBN: 978-0-12-800287-2 © Copyright 2014 Elsevier Ltd. Academic Press Advances in CephalopodAuthor's Science:Biology, personal Ecology, copy Cultivation and Fisheries,Vol 67 (2014) CHAPTER TWO Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries Paul G.K. -

Squids, Octopuses and Lots of Ink
Squids, octopuses and lots of ink Rodrigo B. Salvador1 & Carlo M. Cunha2 1 Staatliches Museum für Naturkunde Stuttgart; Stuttgart, Germany. Eberhard Karls Universität Tübingen; Tübingen, Germany. Email: [email protected] 2 Museu de Zoologia da Universidade de São Paulo; São Paulo, Brazil. Email: [email protected] Splatoon was recently released (second the class Cephalopoda belongs to the phylum quarter of 2015) for the Wii U, receiving a warm Mollusca. welcome by Nintendo fans (it’s nigh unthinkable Cephalopoda is a group that contains a vast for the company to launch a new IP like this) and array of marine animals. Besides squids and generating a flood of fan art on the Internet. The octopuses, it counts with cuttlefish, nautiloids game is a third-person shooter with ink instead and the fossil belemnites and ammonoids. of bullets. It features two races, inklings (the Today, cephalopods are found everywhere in playable one) and octarians (the enemies), and the sea, from the polar regions to the tropics and revolves around the fierce dispute against each from the surface to depths over 5,000 m. There other. (In multiplayer though, its inkling against are over 800 known living species of inkling.) Inklings and octarians (especially the cephalopods, but the fossil record counts with elite soldiers called “octolings”) are based, more than 17,000 species (Boyle & Rodhouse, respectively, on squids and octopuses (Fig. 1), 2005; Rosenberg, 2014). two of the most awesome kinds of animals out The class appeared over 450 million years there. ago during the late Cambrian, the first period of These animals are mollusks, and, more the Paleozoic era (Boyle & Rodhouse, 2005; specifically, cephalopods. -

Ommastrephidae 199
click for previous page Decapodiformes: Ommastrephidae 199 OMMASTREPHIDAE Flying squids iagnostic characters: Medium- to Dlarge-sized squids. Funnel locking appara- tus with a T-shaped groove. Paralarvae with fused tentacles. Arms with biserial suckers. Four rows of suckers on tentacular clubs (club dactylus with 8 sucker series in Illex). Hooks never present hooks never on arms or clubs. One of the ventral pair of arms present usually hectocotylized in males. Buccal connec- tives attach to dorsal borders of ventral arms. Gladius distinctive, slender. funnel locking apparatus with Habitat, biology, and fisheries: Oceanic and T-shaped groove neritic. This is one of the most widely distributed and conspicuous families of squids in the world. Most species are exploited commercially. Todarodes pacificus makes up the bulk of the squid landings in Japan (up to 600 000 t annually) and may comprise at least 1/2 the annual world catch of cephalopods.In various parts of the West- ern Central Atlantic, 6 species of ommastrephids currently are fished commercially or for bait, or have a potential for exploitation. Ommastrephids are powerful swimmers and some species form large schools. Some neritic species exhibit strong seasonal migrations, wherein they occur in huge numbers in inshore waters where they are accessable to fisheries activities. The large size of most species (commonly 30 to 50 cm total length and up to 120 cm total length) and the heavily mus- cled structure, make them ideal for human con- ventral view sumption. Similar families occurring in the area Onychoteuthidae: tentacular clubs with claw-like hooks; funnel locking apparatus a simple, straight groove. -

Life History of the Neon Flying Squid: Effect of the Oceanographic Regime
Vol. 378: 1–11, 2009 MARINE ECOLOGY PROGRESS SERIES Published March 12 doi: 10.3354/meps07873 Mar Ecol Prog Ser OPENPEN ACCESSCCESS FEATURE ARTICLE Life history of the neon flying squid: effect of the oceanographic regime in the North Pacific Ocean Taro Ichii1,*, Kedarnath Mahapatra2, Mitsuo Sakai1, Yoshihiro Okada3 1National Research Institute of Far Seas Fisheries, 2-12-4 Fukuura, Kanazawa, Yokohama-city, Kanagawa 236-8648, Japan 2Tokai University Frontier Ocean Research Center (T-FORCE), 3-20-1 Orido, Shimizu-ward, Shizuoka-city, Shizuoka 424-8610, Japan 3School of Marine Science and Technology, Tokai University, 3-20-1 Orido, Shimizu-ward, Shizuoka-city, Shizuoka 424-8610, Japan ABSTRACT: The North Pacific Ocean population of the neon flying squid Ommastrephes bartramii, which un- dertakes seasonal north–south migrations, consists of autumn and winter–spring spawning cohorts. We ex- amined life history differences between the 2 cohorts in relation to the oceanographic environment. The differ- ences could be explained by seasonal north–south movements of the following 2 oceanographic zones: (1) the optimum spawning zone defined by sea surface temperatures; and (2) the food-rich zone defined by the position of the transition zone chlorophyll front (TZCF). The 2 cohorts use the food-rich zone in different phases of their life cycles. The spawning grounds for the au- Hatchling of the neon flying squid Ommastrephes bartramii tumn cohort occur within the subtropical frontal zone Photo: M. Sakai (STFZ), characterized by enhanced productivity in win- ter due to its proximity to the TZCF, whereas the spawning grounds for the winter–spring cohort occur within the subtropical domain, which is less productive. -

Detection of Potential Fishing Zones for Neon Flying Squid Based on Remote-Sensing Data in the Northwest Pacific Ocean Using an Artificial Neural Network
International Journal of Remote Sensing ISSN: 0143-1161 (Print) 1366-5901 (Online) Journal homepage: http://www.tandfonline.com/loi/tres20 Detection of potential fishing zones for neon flying squid based on remote-sensing data in the Northwest Pacific Ocean using an artificial neural network Jintao Wang, Wei Yu, Xinjun Chen, Lin Lei & Yong Chen To cite this article: Jintao Wang, Wei Yu, Xinjun Chen, Lin Lei & Yong Chen (2015) Detection of potential fishing zones for neon flying squid based on remote-sensing data in the Northwest Pacific Ocean using an artificial neural network, International Journal of Remote Sensing, 36:13, 3317-3330, DOI: 10.1080/01431161.2015.1042121 To link to this article: http://dx.doi.org/10.1080/01431161.2015.1042121 Published online: 01 Jul 2015. Submit your article to this journal Article views: 135 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tres20 Download by: [University of Maine - Orono] Date: 17 August 2016, At: 12:56 International Journal of Remote Sensing, 2015 Vol. 36, No. 13, 3317–3330, http://dx.doi.org/10.1080/01431161.2015.1042121 Detection of potential fishing zones for neon flying squid based on remote-sensing data in the Northwest Pacific Ocean using an artificial neural network Jintao Wanga,b,c,d,WeiYua,b, Xinjun Chena,b,c,d*, Lin Leia,b,c,d, and Yong Chenb,e aCollege of Marine Sciences, Shanghai Ocean University, Shanghai 201306, China; bCollaborative Innovation Center for -
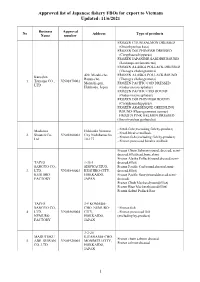
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

Beak Growth Pattern of Purpleback Flying Squid Sthenoteuthis Oualaniensis in the Eastern Tropical Pacific Equatorial Waters
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/273483351 Beak growth pattern of purpleback flying squid Sthenoteuthis oualaniensis in the eastern tropical Pacific equatorial waters Article in Fisheries Science · March 2015 DOI: 10.1007/s12562-015-0857-8 CITATIONS READS 2 100 6 authors, including: Zhou Fang Xinjun Chen Shanghai Ocean University Shanghai Ocean University 22 PUBLICATIONS 36 CITATIONS 170 PUBLICATIONS 700 CITATIONS SEE PROFILE SEE PROFILE Bilin Liu Yong Chen Shanghai Ocean University University of Maine 38 PUBLICATIONS 268 CITATIONS 214 PUBLICATIONS 2,226 CITATIONS SEE PROFILE SEE PROFILE All in-text references underlined in blue are linked to publications on ResearchGate, Available from: Zhou Fang letting you access and read them immediately. Retrieved on: 28 August 2016 Fish Sci DOI 10.1007/s12562-015-0857-8 ORIGINAL ARTICLE Biology Beak growth pattern of purpleback flying squid Sthenoteuthis oualaniensis in the eastern tropical Pacific equatorial waters Zhou Fang · Luoliang Xu · Xinjun Chen · Bilin Liu · Jianhua Li · Yong Chen Received: 29 August 2014 / Accepted: 13 December 2014 © Japanese Society of Fisheries Science 2015 Abstract Cephalopod beaks maintain a stable morphol- length, and lower lateral wall length, which showed lin- ogy, implying that they can be used to explore ecological ear relationships with ML. The relationships between BW influences on squid life history. Understanding the beak and the six beak variables were best fitted with power growth pattern can help us to improve knowledge of the functions, and these functions can be used to estimate trophic characteristics of squids and to estimate squid squid biomass from beak variable values.