Myocardial Ischemia Reperfusion Injury Is Alleviated by Curcumin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research on the Tourism Space Structure in Lingui District of Guilin Based on the Development and Utilization of Landscape Resources
E3S Web of Conferences 53, 03061 (2018) https://doi.org/10.1051/e3sconf/20185303061 ICAEER 2018 Research on the Tourism Space Structure in Lingui District of Guilin Based on the Development and Utilization of Landscape Resources Zhengmin Wen1,2,*,Jie Shi2,Shuangbao Qian2 and Qing Xu2 1Architecture College, Xi'an University of Architecture and Technology, Xi'an, 710055,Cnina; 2School of Civil Engineering and Architecture, Guilin University of Technology, Guilin, 541004, China Abstract: The 257 scenic spots in Lingui covered 5 main categories, 14 sub-categories and 26 basic types, in which there were 33 natural scenic spots and 224 humanistic scenic spots, featured by rich resources stock, humanistic landscape resources-based, and significant space agglomeration; the quality levels are 11 high-quality scenic spots that most of them have been developed, 57 good scenic spots and 189 ordinary scenic spots that have big development potential; of the 36 scenic spots developed so far, 7 are natural, and 29 are humanistic(22 of them have been oriented by cultural relic protection sites); from initial scattered-point to intensive scattered-point to point-axis period, they showed stepped and multi-center structure situation. We found that: 1) five levels of growth pole have been formed; 2) The influence mechanism of development on tourism space is: the theme park is the greatest, the natural landscape resources is secondary and the cultural landscape resources is the least, the former-residence -type cultural relics protection sites and traditional villages have a certain influence, and the influence of high-level landscape resources is big in general. -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

Anisotropic Patterns of Liver Cancer Prevalence in Guangxi in Southwest China: Is Local Climate a Contributing Factor?
DOI:http://dx.doi.org/10.7314/APJCP.2015.16.8.3579 Anisotropic Patterns of Liver Cancer Prevalence in Guangxi in Southwest China: Is Local Climate a Contributing Factor? RESEARCH ARTICLE Anisotropic Patterns of Liver Cancer Prevalence in Guangxi in Southwest China: Is Local Climate a Contributing Factor? Wei Deng1&, Long Long2&*, Xian-Yan Tang3, Tian-Ren Huang1, Ji-Lin Li1, Min- Hua Rong1, Ke-Zhi Li1, Hai-Zhou Liu1 Abstract Geographic information system (GIS) technology has useful applications for epidemiology, enabling the detection of spatial patterns of disease dispersion and locating geographic areas at increased risk. In this study, we applied GIS technology to characterize the spatial pattern of mortality due to liver cancer in the autonomous region of Guangxi Zhuang in southwest China. A database with liver cancer mortality data for 1971-1973, 1990-1992, and 2004-2005, including geographic locations and climate conditions, was constructed, and the appropriate associations were investigated. It was found that the regions with the highest mortality rates were central Guangxi with Guigang City at the center, and southwest Guangxi centered in Fusui County. Regions with the lowest mortality rates were eastern Guangxi with Pingnan County at the center, and northern Guangxi centered in Sanjiang and Rongshui counties. Regarding climate conditions, in the 1990s the mortality rate of liver cancer positively correlated with average temperature and average minimum temperature, and negatively correlated with average precipitation. In 2004 through 2005, mortality due to liver cancer positively correlated with the average minimum temperature. Regions of high mortality had lower average humidity and higher average barometric pressure than did regions of low mortality. -

World Bank Document
Document of The World Bank FOR OFFICIAL USE ONLY Public Disclosure Authorized Report No: PAD809 INTERNATIONAL BANK FOR RECONSTRUCTION AND DEVELOPMENT PROJECT APPRAISAL DOCUMENT ON A PROPOSED LOAN Public Disclosure Authorized IN THE AMOUNT OF US$ 100 MILLION TO THE PEOPLE'S REPUBLIC OF CHINA FOR A GUILIN INTEGRATED ENVIRONMENT MANAGEMENT PROJECT Public Disclosure Authorized January 8, 2015 Water Global Practice East Asia and Pacific Region This document has a restricted distribution and may be used by recipients only in the Public Disclosure Authorized performance of their official duties. Its contents may not otherwise be disclosed without World Bank authorization. CURRENCY EQUIVALENTS (Exchange Rate Effective May 30, 2014) Currency Unit = RMB US$ = 6.17 RMB FISCAL YEAR January 1 – December 31 ABBREVIATIONS AND ACRONYMS AFD French Agency for Development AWTP Average Willingness to Pay CCTV Close Circuit Television CPS Country Partnership Strategy DA Designated Account EA Environmental Assessment EAMMO Environmental Automatic Monitoring Management Office EMP Environmental Management Plan ERR Economic Rate of Return ESMF Environmental and Social Management Framework FMM Financial Management Manual GAO Guangxi Zhuang Autonomous Region Audit Office GEPB Guilin Municipal Environmental Protection Bureau GDRC Guilin Municipal Development Reform Commission GFB Guilin Municipal Finance Bureau GMG Guilin Municipal Government GPUB Guilin Public Utilities Bureau GSC Guilin Sewerage Company GWSC Guilin Water Supply Company GFD Guangxi Zhuang Autonomous -

Guilin Dynamic & Scenic 4-Day Trip
High Speed Rail: Guilin Dynamic & Scenic 4-Day Trip Day 1 Itinerary Suggested Transportation Hong Kong → Guilin [Hong Kong West Kowloon Station → High Speed Rail Guilinxi Railway Station] For shopping and dining convenience, you can stay in a hotel in the Taxi: city centre. Or you might choose a hotel located next to the river if Take a 22-minute taxi ride from you like the scenery of Li River. Guilinxi Railway Station Hotel for reference: Sheraton Guilin Hotel Address: 15 Binjiang Road, Xiufeng District, Guilin Enjoy afternoon tea at a restaurant near the hotel On foot: Walk for about 7 minutes from the Restaurant for reference: Miss Yin Dessert hotel. Address: 5 Shanhu North Road, Xiufeng District, Guilin Shine & Glitter - Sun & Moon Twin Pagodas On foot: Walk for about 10 minutes from Located in the city centre, the Sun and Moon Twin Pagodas are close the restaurant. to the famous Elephant Trunk Hill Scenic Area. The 41-metre high Sun Pagoda has nine levels, all decorated with pure copper. Made up of 350 tons of copper, it has set three world records: the world's tallest copper building, the world's tallest water pagoda and the world's tallest copper pagoda. Next to it is the 35-metre high Moon Pagoda with a total of seven levels. Also known as the Glass Pagoda, the primarily silver-coloured pagoda has aesthetically carved and painted windows with doors on every level. It is recommended to start the tour at dusk so that you can enjoy the view in daylight and later also admire the view at night when the two pagodas are lit up. -

中國內地指定醫院列表 出版日期: 2019 年 7 月 1 日 Designated Hospital List in Mainland China Published Date: 1 Jul 2019
中國內地指定醫院列表 出版日期: 2019 年 7 月 1 日 Designated Hospital List in Mainland China Published Date: 1 Jul 2019 省 / 自治區 / 直轄市 醫院 地址 電話號碼 Provinces / 城市/City Autonomous Hospital Address Tel. No. Regions / Municipalities 中國人民解放軍第二炮兵總醫院 (第 262 醫院) 北京 北京 西城區新街口外大街 16 號 The Second Artillery General Hospital of Chinese 10-66343055 Beijing Beijing 16 Xinjiekou Outer Street, Xicheng District People’s Liberation Army 中國人民解放軍總醫院 (第 301 醫院) 北京 北京 海澱區復興路 28 號 The General Hospital of Chinese People's Liberation 10-82266699 Beijing Beijing 28 Fuxing Road, Haidian District Army 北京 北京 中國人民解放軍第 302 醫院 豐台區西四環中路 100 號 10-66933129 Beijing Beijing 302 Military Hospital of China 100 West No.4 Ring Road Middle, Fengtai District 中國人民解放軍總醫院第一附屬醫院 (中國人民解 北京 北京 海定區阜成路 51 號 放軍 304 醫院) 10-66867304 Beijing Beijing 51 Fucheng Road, Haidian District PLA No.304 Hospital 北京 北京 中國人民解放軍第 305 醫院 西城區文津街甲 13 號 10-66004120 Beijing Beijing PLA No.305 Hospital 13 Wenjin Street, Xicheng District 北京 北京 中國人民解放軍第 306 醫院 朝陽區安翔北里 9 號 10-66356729 Beijing Beijing The 306th Hospital of PLA 9 Anxiang North Road, Chaoyang District 中國人民解放軍第 307 醫院 北京 北京 豐台區東大街 8 號 The 307th Hospital of Chinese People’s Liberation 10-66947114 Beijing Beijing 8 East Street, Fengtai District Army 中國人民解放軍第 309 醫院 北京 北京 海澱區黑山扈路甲 17 號 The 309th Hospital of Chinese People’s Liberation 10-66775961 Beijing Beijing 17 Heishanhu Road, Haidian District Army 中國人民解放軍第 466 醫院 (空軍航空醫學研究所 北京 北京 海澱區北窪路北口 附屬醫院) 10-81988888 Beijing Beijing Beiwa Road North, Haidian District PLA No.466 Hospital 北京 北京 中國人民解放軍海軍總醫院 (海軍總醫院) 海澱區阜成路 6 號 10-66958114 Beijing Beijing PLA Naval General Hospital 6 Fucheng Road, Haidian District 北京 北京 中國人民解放軍空軍總醫院 (空軍總醫院) 海澱區阜成路 30 號 10-68410099 Beijing Beijing Air Force General Hospital, PLA 30 Fucheng Road, Haidian District 中華人民共和國北京市昌平區生命園路 1 號 北京 北京 北京大學國際醫院 Yard No.1, Life Science Park, Changping District, Beijing, 10-69006666 Beijing Beijing Peking University International Hospital China, 東城區南門倉 5 號(西院) 5 Nanmencang, Dongcheng District (West Campus) 北京 北京 北京軍區總醫院 10-66721629 Beijing Beijing PLA. -

World Bank Document
E4566 v3 REV Public Disclosure Authorized Guilin Integrated Environment Management Project of the World Bank Funded Public Disclosure Authorized Comprehensive Environment Impact Assessment Report Public Disclosure Authorized Prepared by: The Environment Protection Science Academy of Public Disclosure Authorized Guangxi Zhuang Autonomous Region Certificate No.: Guohuanpinzheng Jiazi No. 2902 Date: June 2014 Comprehensive EIA Report Guilin Integrated Table of Contents Environment Management Project of the World Bank Funded Table of Contents ABBREVIATIONS ............................................................................................................................................... 1 1 FOREWORD ...................................................................................................................................................... 2 1.1 OVERALL BACKGROUND OF THE PROJECT ................................................................................................ 2 1.2 OVERVIEW OF THE COMPREHENSIVE ENVIRONMENT ASSESSMENT REPORT .......................................... 3 1.3 SCOPE AND TIME SECTION OF ENVIRONMENT ASSESSMENT, AND THE ENVIRONMENT PROTECTION TARGETS ............................................................................................................................................................ 5 1.4 ENVIRONMENTAL IMPACT FACTORS AND ASSESSMENT FACTORS ........................................................... 7 1.5 ENVIRONMENTAL POLICY AND RULE DOCUMENTS .................................................................................. -

QUANTITATIVE REMOTE SENSING ANALYSIS of THERMAL ENVIRONMENT CHANGES in the MAIN URBAN AREA of Guilin BASED on GEE
The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Volume XLII-3/W10, 2020 International Conference on Geomatics in the Big Data Era (ICGBD), 15–17 November 2019, Guilin, Guangxi, China QUANTITATIVE REMOTE SENSING ANALYSIS OF THERMAL ENVIRONMENT CHANGES IN THE MAIN URBAN AREA OF Guilin BASED ON GEE Peiqing Lou, Bolin Fu*, Xingchen Lin, Tingyuan Tang, Lu Bi College of Geomatics and Geoinformation, Guilin University of Technology, Guilin 541004 [email protected] (L.P.); [email protected] (F.B.); [email protected] (L.X.); [email protected] (T.T.); [email protected] (B.L.) Commission VI, WG VI/4 KEY WORDS: Thermal Environment, GEE, Mono-window Algorithm, Random Forest Algorithm, Landsat 8, Dynamic Analysis ABSTRACT: The dynamic change of urban thermal environment caused by the change of land use type has become one of the important problems of urban ecological environment protection. In Guilin city as research area, based on the Google Earth Engine (GEE), the random forest algorithm was used to classify the land use classification of Landsat remote sensing images in 2010, 2014 and 2018, and the mono-window algorithm was used to calculate the surface temperature. The surface vegetation was solved according to the NDVI pixel binary model. Coverage, and finally dynamic statistics and comparative analysis of land use, vegetation cover and surface temperature. The main results as follows. (1) From 2010 to 2018, the average temperature in the main urban area of Guilin is on the rise (increased by 1.29 °C), and the temperature zones in each class are converted from low temperature zone, lower temperature zone and medium temperature zone to higher temperature zone and high temperature zone. -

Research Article Marker Genes Change of Synovial Fibroblasts in Rheumatoid Arthritis Patients
Hindawi BioMed Research International Volume 2021, Article ID 5544264, 17 pages https://doi.org/10.1155/2021/5544264 Research Article Marker Genes Change of Synovial Fibroblasts in Rheumatoid Arthritis Patients Lifen Liao,1 Ke Liang,2 Lan Lan,1 Jinheng Wang,1 and Jun Guo 3 1Department of Laboratory, Affiliated Hospital of Guilin Medical University, Xiufeng District, Guilin, 541001 Guangxi, China 2Department of Laboratory, Nanxishan Hospital of Guangxi Zhuang Autonomous Region, Xiangshan District, Guilin, 541002 Guangxi, China 3Department of Hematology, Rizhao People’s Hospital, Donggang District, Rizhao, 276800 Shandong, China Correspondence should be addressed to Jun Guo; [email protected] Received 27 February 2021; Revised 14 May 2021; Accepted 24 May 2021; Published 7 June 2021 Academic Editor: Tao Huang Copyright © 2021 Lifen Liao et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Rheumatoid arthritis (RA) is a chronic condition that manifests as inflammation of synovial joints, leading to joint destruction and deformity. Methods. We identified single-cell RNA-seq data of synovial fibroblasts from RA and osteoarthritis (OA) patients in GSE109449 dataset. RA- and OA-specific cellular subpopulations were identified, and enrichment analysis was performed. Further, key genes for RA and OA were obtained by combined analysis with differentially expressed genes (DEGs) between RA and OA in GSE56409 dataset. The diagnostic role of key genes for RA was predicted using receiver operating characteristic (ROC) curve. Finally, we identified differences in immune cell infiltration between RA and OA patients, and utilized flow cytometry, qRT-PCR, and Western blot were used to examine the immune cell and key genes in RA patients. -
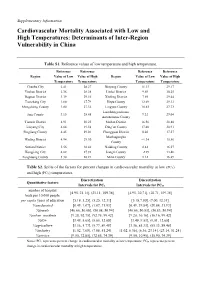
Cardiovascular Mortality Associated with Low and High Temperatures: Determinants of Inter-Region Vulnerability in China
Supplementary Information Cardiovascular Mortality Associated with Low and High Temperatures: Determinants of Inter-Region Vulnerability in China Table S1. Reference values of low temperature and high temperature. Reference Reference Reference Reference Region Value of Low Value of High Region Value of Low Value of High Temperature Temperature Temperature Temperature Chaohu City 2.41 28.27 Binyang County 11.13 29.17 Yushan District 2.36 28.28 Liubei District 9.69 30.25 Daguan District 3.39 29.16 Xiufeng District 7.89 29.44 Tianchang City 1.80 27.79 Hepu County 12.69 29.33 Mengcheng County 1.00 27.32 Lingyun County 10.82 27.73 Luochengyaolaozu Jing County 3.15 28.48 9.32 29.04 Autonomous County Tianxin District 4.91 30.23 Meilan District 16.56 28.88 Liuyang City 4.64 29.38 Ding’an County 17.40 28.91 Pingjiang County 4.45 29.01 Chengguan District 0.20 17.57 Mozhugongka Wuling District 4.94 29.30 −1.34 15.56 County Suxian District 5.56 30.42 Naidong County 0.42 16.57 Hongjiang City 4.82 27.93 Jiangzi County −2.89 13.48 Fenghuang County 5.30 28.21 Milin County 1.12 16.49 Table S2. Splits of the factors for percent changes in cardiovascular mortality at low (PCL) and high (PCH) temperatures. Discretization Discretization Quantitative factors Intervals for PCL Intervals for PCH number of hospital [4.95, 21.11], (21.11, 109.38] [4.95, 30.71], (30.71, 109.38] beds per 10,000 people per capita years of education [5.18, 5.25], (5.25, 12.31] [5.18,7.00], (7.00, 12.31] %uneducated [0.49, 1.07], (1.07, 31.93] [0.49, 29.84], (29.84, 31.93] %female [46.66, -

Annual Development Report on China's Trademark Strategy 2013
Annual Development Report on China's Trademark Strategy 2013 TRADEMARK OFFICE/TRADEMARK REVIEW AND ADJUDICATION BOARD OF STATE ADMINISTRATION FOR INDUSTRY AND COMMERCE PEOPLE’S REPUBLIC OF CHINA China Industry & Commerce Press Preface Preface 2013 was a crucial year for comprehensively implementing the conclusions of the 18th CPC National Congress and the second & third plenary session of the 18th CPC Central Committee. Facing the new situation and task of thoroughly reforming and duty transformation, as well as the opportunities and challenges brought by the revised Trademark Law, Trademark staff in AICs at all levels followed the arrangement of SAIC and got new achievements by carrying out trademark strategy and taking innovation on trademark practice, theory and mechanism. ——Trademark examination and review achieved great progress. In 2013, trademark applications increased to 1.8815 million, with a year-on-year growth of 14.15%, reaching a new record in the history and keeping the highest a mount of the world for consecutive 12 years. Under the pressure of trademark examination, Trademark Office and TRAB of SAIC faced the difficuties positively, and made great efforts on soloving problems. Trademark Office and TRAB of SAIC optimized the examination procedure, properly allocated examiners, implemented the mechanism of performance incentive, and carried out the “double-points” management. As a result, the Office examined 1.4246 million trademark applications, 16.09% more than last year. The examination period was maintained within 10 months, and opposition period was shortened to 12 months, which laid a firm foundation for performing the statutory time limit. —— Implementing trademark strategy with a shift to effective use and protection of trademark by law. -
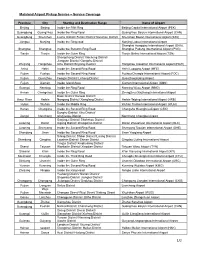
Mainland Airport Pickup Service – Service Coverage
Mainland Airport Pickup Service – Service Coverage Province City Starting and Destination Range Name of Airport Beijing Beijing Inside the Fifth Ring Beijing Capital International Airport (PEK) Guangdong Guangzhou Inside the Ring Road Guangzhou Baiyun International Airport (CAN) Guangdong Shenzhen Luohu District/ Futian District/ Nanshan District Shenzhen Baoan International Airport (SZX) Jiangsu Nanjing Inside the Ring road Nanjing Lukou International Airport Shanghai Hongqiao International Airport (SHA), Shanghai Shanghai Inside the Suburbs Ring Road Shanghai Pudong International Airport (PVG) Tianjin Tianjin Inside the Outer Ring Tianjin Binhai International Airport (TSN) Shangcheng District/ Xiacheng District/ Jianggan District/ Gongshu District/ Zhejiang Hangzhou Xihu District/ Binjiang Dustrict Hangzhou Xiaoshan International Airport (HGH) Anhui Hefei Inside the Second Ring Road Hefei Luogang Airport (HFE) Fujian Fuzhou Inside the Second Ring Road Fuzhou Changle International Airport (FOC) Fujian Quanzhou Fengze District/ Licheng District Quanzhoujinjiang Airport Fujian Xiamen Inside Island Area Xiamen International Airport (XMN) Guangxi Nanning Inside the Ring Road Nanning Wuxu Airport (NNG) Henan Chengchou Inside the Outer Ring Zhengzhou Xinzheng International Airport Daoli District/ Daowai District/ Amur River Harbin Nangang District/ Xiangfang District Harbin Taiping International Airport (HRB) Hubei Wuhan Inside the Middle Ring Wuhan Tianhe International Airport (WUH) Hunan Changsha Inside the Second Ring Road Changsha Huanghua