Curriculum Vitae: Daniel J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BAFF-Neutralizing Interaction of Belimumab Related to Its Therapeutic Efficacy for Treating Systemic Lupus Erythematosus
ARTICLE DOI: 10.1038/s41467-018-03620-2 OPEN BAFF-neutralizing interaction of belimumab related to its therapeutic efficacy for treating systemic lupus erythematosus Woori Shin1, Hyun Tae Lee1, Heejin Lim1, Sang Hyung Lee1, Ji Young Son1, Jee Un Lee1, Ki-Young Yoo1, Seong Eon Ryu2, Jaejun Rhie1, Ju Yeon Lee1 & Yong-Seok Heo1 1234567890():,; BAFF, a member of the TNF superfamily, has been recognized as a good target for auto- immune diseases. Belimumab, an anti-BAFF monoclonal antibody, was approved by the FDA for use in treating systemic lupus erythematosus. However, the molecular basis of BAFF neutralization by belimumab remains unclear. Here our crystal structure of the BAFF–belimumab Fab complex shows the precise epitope and the BAFF-neutralizing mechanism of belimumab, and demonstrates that the therapeutic activity of belimumab involves not only antagonizing the BAFF–receptor interaction, but also disrupting the for- mation of the more active BAFF 60-mer to favor the induction of the less active BAFF trimer through interaction with the flap region of BAFF. In addition, the belimumab HCDR3 loop mimics the DxL(V/L) motif of BAFF receptors, thereby binding to BAFF in a similar manner as endogenous BAFF receptors. Our data thus provides insights for the design of new drugs targeting BAFF for the treatment of autoimmune diseases. 1 Department of Chemistry, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Republic of Korea. 2 Department of Bio Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea. These authors contributed equally: Woori Shin, Hyun Tae Lee, Heejin Lim, Sang Hyung Lee. -
(12) United States Patent (10) Patent No.: US 7,074,961 B2 Wang Et Al
US007074961B2 (12) United States Patent (10) Patent No.: US 7,074,961 B2 Wang et al. (45) Date of Patent: Jul. 11, 2006 (54) ANTIDEPRESSANTS AND THEIR 6.211,171 B1 4/2001 Sawynok et al. ANALOGUES AS LONG-ACTING LOCAL 6,545,057 B1 4/2003 Wang et al. ANESTHETCS AND ANALGESCS 2001/0036943 A1 11/2001 Coe et al. (75) Inventors: Ging Kuo Wang, Westwood, MA (US); FOREIGN PATENT DOCUMENTS Peter Gerner, Weston, MA (US); Donald K. Verrecchia, Winchester, MA CH 534124 A 2, 1973 (US) WO WO95/17903 A1 7, 1995 WO WO95/1818.6 A1 7, 1995 (73) Assignee: The Brigham and Women's Hospital, WO WO 99.59.598 A1 11, 1999 Inc., Boston, MA (US) WO WO O2/060870 A2 8, 2002 (*) Notice: Subject to any disclaimer, the term of this OTHER PUBLICATIONS patent is extended or adjusted under 35 U.S.C. 154(b) by 451 days. Luo et al., Xenobiotica, vol. 25, No. 3, pp. 291-301, 1995.* (21) Appl. No.: 10/117,708 Ehlert et al., The Interaction of Amitriptyline, Doxepin, Imipramine and Their N-Methyl Quaternary Ammonium (22) Filed: Apr. 4, 2002 Derivatives with Subtypes of Muscarinic Receptors in Brain (65) Prior Publication Data and Heart, The Journal of Pharmacology and Experimental Therapeutics, Apr. 1990, vol. 253, No. 1, pp. 13–19. US 2003/00968.05 A1 May 22, 2003 Gerner, P., et al., Anesthesiology (2002) 96:1435–42. Related U.S. Application Dat e pplication Uata Khan, M.A., et al., Anesthesiology (2002) 96:109–16. (63) stripps'965,138, filed on Sep. -

Ocrevus (Ocrelizumab) Policy Number: C11250-A
Prior Authorization Criteria Ocrevus (ocrelizumab) Policy Number: C11250-A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DUE BY OR BEFORE 8/1/2017 2/17/2021 4/26/2022 LAST P&T J CODE TYPE OF CRITERIA APPROVAL/VERSION J2350-injection,ocrelizumab, Q2 2021 RxPA 1mg 20200428C11250-A PRODUCTS AFFECTED: Ocrevus (ocrelizumab) DRUG CLASS: Multiple Sclerosis Agents - Monoclonal Antibodies ROUTE OF ADMINISTRATION: Intravenous PLACE OF SERVICE: Specialty Pharmacy or Buy and Bill The recommendation is that medications in this policy will be for pharmacy benefit coverage and the IV infusion products administered in a place of service that is a non-hospital facility-based location (i.e., home infusion provider, provider’s office, free-standing ambulatory infusion center) AVAILABLE DOSAGE FORMS: Ocrevus SOLN 300MG/10ML FDA-APPROVED USES: Indicated for the treatment of: • Relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing- remitting disease, and active secondary progressive disease, in adults • Primary progressive MS, in adults COMPENDIAL APPROVED OFF-LABELED USES: None COVERAGE CRITERIA: INITIAL AUTHORIZATION DIAGNOSIS: Multiple Sclerosis REQUIRED MEDICAL INFORMATION: A. RELAPSING FORMS OF MULTIPLE SCLEROSIS: 1. Documentation of a definitive diagnosis of a relapsing form of multiple sclerosis as defined by the McDonald criteria (see Appendix), including: Relapsing- remitting multiple sclerosis [RRMS], secondary-progressive multiple sclerosis [SPMS] with relapses, and progressive- relapsing multiple sclerosis [PRMS] or First clinical episode with MRI features consistent with multiple sclerosis Molina Healthcare, Inc. confidential and proprietary © 2021 This document contains confidential and proprietary information of Molina Healthcare and cannot be reproduced, distributed, or printed without written permission from Molina Healthcare. -

Exploring the Association Between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: a Vigibase Study
Drug Safety https://doi.org/10.1007/s40264-018-00789-9 ORIGINAL RESEARCH ARTICLE Exploring the Association between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: A VigiBase Study Lotte A. Minnema1,2 · Thijs J. Giezen2,3 · Patrick C. Souverein1 · Toine C. G. Egberts1,4 · Hubert G. M. Leufkens1 · Helga Gardarsdottir1,4,5 © The Author(s) 2019 Abstract Introduction Several monoclonal antibodies (mAbs) have been linked to neuropsychiatric adverse efects in patients, includ- ing depression and suicidal ideation and behavior. Objective The aim of this study was to quantify and characterize spontaneously reported adverse drug reactions (ADRs) of depression and suicidal ideation and behavior related to mAb users, and to explore a possible association with their mecha- nism of action. Methods We included mAb ADRs that were reported in VigiBase, and identifed those related to depression and suicidal ideation and behavior. Reporting odds ratios (RORs) were estimated for each mAb (bevacizumab as the reference) and according to their infuence on the immune system (not directly targeting [reference], stimulating, or suppressing). Those suppressing the immune system were further divided into their intended indication (auto-immune diseases, cancer). Results Overall, 2,924,319 ADRs for 44 mAbs were included; 9455 ADRs were related to depression and 1770 were related to suicidal ideation and behavior. The association was strongest for natalizumab and belimumab, both for depression (ROR 5.7, 95% confdence interval [CI] 5.0–6.4; and ROR 5.1, 95% CI 4.2–6.2) and suicidal ideation and behavior (ROR 12.0, 95% CI 7.9–18.3; and ROR 20.2, 95% CI 12.4–33.0). -

Fontolizumab, a Humanised Anti-Interferon C Antibody
1131 INFLAMMATORY BOWEL DISEASE Gut: first published as 10.1136/gut.2005.079392 on 28 February 2006. Downloaded from Fontolizumab, a humanised anti-interferon c antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease D W Hommes, T L Mikhajlova, S Stoinov, D Sˇtimac, B Vucelic, J Lonovics, M Za´kuciova´, G D’Haens, G Van Assche, S Ba, S Lee, T Pearce ............................................................................................................................... Gut 2006;55:1131–1137. doi: 10.1136/gut.2005.079392 See end of article for Introduction: Interferon c is a potent proinflammatory cytokine implicated in the inflammation of Crohn’s authors’ affiliations ....................... disease (CD). We evaluated the safety and efficacy of fontolizumab, a humanised anti-interferon c antibody, in patients with moderate to severe CD. Correspondence to: Methods: A total of 133 patients with Crohn’s disease activity index (CDAI) scores between 250 and 450, Dr D W Hommes, Department inclusive, were randomised to receive placebo or fontolizumab 4 or 10 mg/kg. Forty two patients Gastroenterology and received one dose and 91 patients received two doses on days 0 and 28. Investigators and patients were Hepatology, Academic unaware of assignment. Study end points were safety, clinical response (decrease in CDAI of 100 points or Medical Centre, C2-111, ( Meibergdreef 9 1105 AZ, more), and remission (CDAI 150). Amsterdam, the Results: There was no statistically significant difference in the primary end point of the study (clinical Netherlands; response) between the fontolizumab and placebo groups after a single dose at day 28. However, patients d.w.hommes@ receiving two doses of fontolizumab demonstrated doubling in response rate at day 56 compared with amc.uva.nl placebo: 32% (9/28) versus 69% (22/32, p = 0.02) and 67% (21/31, p = 0.03) for the placebo, and 4 Revised version received and 10 mg/kg fontolizumab groups, respectively. -

RESI Boston Program Guide 09-26-2017 Digital
SEPTEMBER 26 , 2017 BOSTON, MA Early stage investors, fundraising CEOs, scientist-entrepreneurs, strategic partners, and service providers now have an opportunity to Make a Compelling Connection ONSITE GUIDE LIFE SCIENCE NATION Connecting Products, Services & Capital #RESIBOS17 | RESIConference.com | Boston Marriott Copley Place FLOOR PLAN Therapeutics Track 2 Investor Track 3 & track4 Track 1 Device, Panels Workshops & Diagnostic & HCIT Asia Investor Panels Panels Ad-Hoc Meeting Area Breakfast & Lunch DINING 29 25 30 26 31 27 32 28 33 29 34 30 35 Breakfast / LunchBreakfast BUFFETS 37 28 24 27 23 26 22 25 21 24 20 23 19 22 exhibit hall 40 15 13 16 14 17 15 18 16 19 17 20 18 21 39 INNOVATION 14 12 13 11 12 10 11 9 10 8 9 7 8 EXHIBITORS CHALLENGE 36 38 FINALISTS 1 1 2 2 3 3 4 4 5 5 6 6 7 Partnering Check-in PARTNERING Forum Lunch BUFFETS Breakfast / Breakfast RESTROOM cocktail reception REGISTRATION content Welcome to RESI - - - - - - - - - - - - - - - 2 RESI Agenda - - - - - - - - - - - - - - - - - - 3 BOSTON RESI Innovation Challenge - - - - - - - 5 Exhibiting Companies - - - - - - - - - - 12 Track 1: Therapeutics Investor Panels - - - - - - - - - - - - - - - 19 Track 2: Device, Diagnostic, & HCIT Investor Panels - - - - 29 Track 3: Entrepreneur Workshops - - - - - - - - - - - - - - - - - - 38 Track 4: Asia-North America Workshop & Panels - - - - - - 41 Track 5: Partnering Forum - - - - - - - - - - - - - - - - - - - - - - - - 45 Sponsors & Media Partners - - - - - - - - - - - - - - - - - - - - - - - 46 1 welcome to resi On behalf of Life Science Nation (LSN) and our title sponsors WuXi AppTec and Johnson & Johnson Innovation JLABS, I would like to thank you for joining us at RESI Boston. LSN is very happy to welcome you all to Boston, the city where it all began, for our 14th RESI event. -

New Biological Therapies: Introduction to the Basis of the Risk of Infection
New biological therapies: introduction to the basis of the risk of infection Mario FERNÁNDEZ RUIZ, MD, PhD Unit of Infectious Diseases Hospital Universitario “12 de Octubre”, Madrid ESCMIDInstituto de Investigación eLibraryHospital “12 de Octubre” (i+12) © by author Transparency Declaration Over the last 24 months I have received honoraria for talks on behalf of • Astellas Pharma • Gillead Sciences • Roche • Sanofi • Qiagen Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Paul Ehrlich (1854-1915) • “side-chain” theory (1897) • receptor-ligand concept (1900) • “magic bullet” theory • foundation for specific chemotherapy (1906) • Nobel Prize in Physiology and Medicine (1908) (together with Metchnikoff) Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author 1981: B-1 antibody (tositumomab) anti-CD20 monoclonal antibody 1997: FDA approval of rituximab for the treatment of relapsed or refractory CD20-positive NHL 2001: FDA approval of imatinib for the treatment of chronic myelogenous leukemia Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Functional classification of targeted (biological) agents • Agents targeting soluble immune effector molecules • Agents targeting cell surface receptors -

Testosterone, Myocardial Function, and Mortality
Heart Failure Reviews https://doi.org/10.1007/s10741-018-9721-0 Testosterone, myocardial function, and mortality Vittorio Emanuele Bianchi1 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract The cardiovascular system is particularly sensitive to androgens, but some controversies exist regarding the effect of testosterone on the heart. While among anabolic abusers, cases of sudden cardiac death have been described, recently it was reported that low serum level of testosterone was correlated with increased risk of cardiovascular diseases (CVD) and mortality rate. This review aims to evaluate the effect of testosterone on myocardial tissue function, coronary artery disease (CAD), and death. Low testosterone level is associated with increased incidence of CAD and mortality. Testosterone administration in hypogonadal elderly men and women has a positive effect on cardiovascular function and improved clinical outcomes and survival time. Although at supraphysiologic doses, androgen may have a toxic effect, and at physiological levels, testosterone is safe and exerts a beneficial effect on myocardial function including mechanisms at cellular and mitochondrial level. The interaction with free testosterone and estradiol should be considered. Further studies are necessary to better understand the interaction mechanisms for an optimal androgen therapy in CVD. Keywords Testosterone . Coronary artery disease . Heart failure . Cardiovascular disease . Cardiomyocytes . Cardiac mitochondria . Cardiovascular mortality Introduction essential role [20]. Asymptomatic hypogonadism is observed in men 30–79 years old with an incidence of about 5.6% [21] The effects of testosterone on myocardial function have gen- and is more relevant in aging subjects. In recent years, pre- erated a great deal of discussion in the literature but remain scriptions for testosterone replacement therapy have increased controversial. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Management Team
Management Team Bruce C. Cozadd Executive Chairman Bruce Cozadd joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Mr. Cozadd served as a consultant to companies in the biopharmaceutical industry. From 1991 until 2001, he held various positions with ALZA Corporation, a pharmaceutical company now owned by Johnson & Johnson, most recently as its Executive Vice President and Chief Operating Officer, with responsibility for research and development, manufacturing and sales and marketing. Previously at ALZA Corporation he held the roles of Chief Financial Officer and Vice President, Corporate Planning and Analysis. Mr. Cozadd received a B.S. from Yale University and an M.B.A. from the Stanford Graduate School of Business. Mr. Cozadd serves on the boards of Cerus Corporation, a biopharmaceutical company; Threshold Pharmaceuticals, a biotechnology company; and The Nueva School and Stanford Hospital and Clinics, both non-profit organizations. Samuel R. Saks, MD Chief Executive Officer Samuel Saks, M.D., joined Jazz Pharmaceuticals at its inception. From 2001 until he joined Jazz Pharmaceuticals, Dr. Saks was Company Group Chairman of ALZA Corporation and served as a member of the Johnson & Johnson Pharmaceutical Group Operating Committee. From 1992 until 2001, he held various positions with ALZA Corporation, most recently as its Chief Medical Officer and Group Vice President, where he was responsible for clinical and commercial activities. Dr. Saks received a B.S. and an M.D. from the University of Illinois. Dr. Saks serves on the board of Trubion Pharmaceuticals and Cougar Biotechnology. Robert M. Myers President Robert Myers joined Jazz Pharmaceuticals at its inception and was appointed as Jazz Pharmaceuticals’ President in March 2007. -

Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis
REVIEW published: 09 July 2021 doi: 10.3389/fimmu.2021.686155 Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis † † Jie Huang 1 , Xuekun Fu 1 , Xinxin Chen 1, Zheng Li 1, Yuhong Huang 1 and Chao Liang 1,2* 1 Department of Biology, Southern University of Science and Technology, Shenzhen, China, 2 Institute of Integrated Bioinfomedicine and Translational Science (IBTS), School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China Rheumatoid arthritis (RA) is a systemic poly-articular chronic autoimmune joint disease that mainly damages the hands and feet, which affects 0.5% to 1.0% of the population worldwide. With the sustained development of disease-modifying antirheumatic drugs (DMARDs), significant success has been achieved for preventing and relieving disease activity in RA patients. Unfortunately, some patients still show limited response to DMARDs, which puts forward new requirements for special targets and novel therapies. Understanding the pathogenetic roles of the various molecules in RA could facilitate discovery of potential therapeutic targets and approaches. In this review, both Edited by: existing and emerging targets, including the proteins, small molecular metabolites, and Trine N. Jorgensen, epigenetic regulators related to RA, are discussed, with a focus on the mechanisms that Case Western Reserve University, result in inflammation and the development of new drugs for blocking the various United States modulators in RA. Reviewed by: Åsa Andersson, Keywords: rheumatoid arthritis, targets, proteins, small molecular metabolites, epigenetic regulators Halmstad University, Sweden Abdurrahman Tufan, Gazi University, Turkey *Correspondence: INTRODUCTION Chao Liang [email protected] Rheumatoid arthritis (RA) is classified as a systemic poly-articular chronic autoimmune joint † disease that primarily affects hands and feet. -
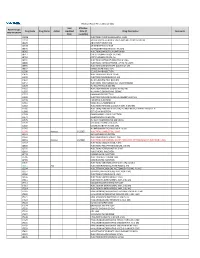
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB,