Ocrevus (Ocrelizumab) Policy Number: C11250-A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BAFF-Neutralizing Interaction of Belimumab Related to Its Therapeutic Efficacy for Treating Systemic Lupus Erythematosus
ARTICLE DOI: 10.1038/s41467-018-03620-2 OPEN BAFF-neutralizing interaction of belimumab related to its therapeutic efficacy for treating systemic lupus erythematosus Woori Shin1, Hyun Tae Lee1, Heejin Lim1, Sang Hyung Lee1, Ji Young Son1, Jee Un Lee1, Ki-Young Yoo1, Seong Eon Ryu2, Jaejun Rhie1, Ju Yeon Lee1 & Yong-Seok Heo1 1234567890():,; BAFF, a member of the TNF superfamily, has been recognized as a good target for auto- immune diseases. Belimumab, an anti-BAFF monoclonal antibody, was approved by the FDA for use in treating systemic lupus erythematosus. However, the molecular basis of BAFF neutralization by belimumab remains unclear. Here our crystal structure of the BAFF–belimumab Fab complex shows the precise epitope and the BAFF-neutralizing mechanism of belimumab, and demonstrates that the therapeutic activity of belimumab involves not only antagonizing the BAFF–receptor interaction, but also disrupting the for- mation of the more active BAFF 60-mer to favor the induction of the less active BAFF trimer through interaction with the flap region of BAFF. In addition, the belimumab HCDR3 loop mimics the DxL(V/L) motif of BAFF receptors, thereby binding to BAFF in a similar manner as endogenous BAFF receptors. Our data thus provides insights for the design of new drugs targeting BAFF for the treatment of autoimmune diseases. 1 Department of Chemistry, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Republic of Korea. 2 Department of Bio Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea. These authors contributed equally: Woori Shin, Hyun Tae Lee, Heejin Lim, Sang Hyung Lee. -

Exploring the Association Between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: a Vigibase Study
Drug Safety https://doi.org/10.1007/s40264-018-00789-9 ORIGINAL RESEARCH ARTICLE Exploring the Association between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: A VigiBase Study Lotte A. Minnema1,2 · Thijs J. Giezen2,3 · Patrick C. Souverein1 · Toine C. G. Egberts1,4 · Hubert G. M. Leufkens1 · Helga Gardarsdottir1,4,5 © The Author(s) 2019 Abstract Introduction Several monoclonal antibodies (mAbs) have been linked to neuropsychiatric adverse efects in patients, includ- ing depression and suicidal ideation and behavior. Objective The aim of this study was to quantify and characterize spontaneously reported adverse drug reactions (ADRs) of depression and suicidal ideation and behavior related to mAb users, and to explore a possible association with their mecha- nism of action. Methods We included mAb ADRs that were reported in VigiBase, and identifed those related to depression and suicidal ideation and behavior. Reporting odds ratios (RORs) were estimated for each mAb (bevacizumab as the reference) and according to their infuence on the immune system (not directly targeting [reference], stimulating, or suppressing). Those suppressing the immune system were further divided into their intended indication (auto-immune diseases, cancer). Results Overall, 2,924,319 ADRs for 44 mAbs were included; 9455 ADRs were related to depression and 1770 were related to suicidal ideation and behavior. The association was strongest for natalizumab and belimumab, both for depression (ROR 5.7, 95% confdence interval [CI] 5.0–6.4; and ROR 5.1, 95% CI 4.2–6.2) and suicidal ideation and behavior (ROR 12.0, 95% CI 7.9–18.3; and ROR 20.2, 95% CI 12.4–33.0). -

Curriculum Vitae: Daniel J
CURRICULUM VITAE: DANIEL J. WALLACE, M.D., F.A.C.P., M.A.C.R. Up to date as of January 1, 2019 Personal: Address: 8750 Wilshire Blvd, Suite 350 Beverly Hills, CA 90211 Phone: (310) 652-0010 FAX: (310) 360-6219 E mail: [email protected] Education: University of Southern California, 2/67-6/70, BA Medicine, 1971. University of Southern California, 9/70-6/74, M.D, 1974. Postgraduate Training: 7/74-6/75 Medical Intern, Rhode Island (Brown University) Hospital, Providence, RI. 7/75-6/77 Medical Resident, Cedars-Sinai Medical Center, Los Angeles, CA. 7/77-6/79 Rheumatology Fellow, UCLA School of Medicine, Los Angeles, CA. Medical Boards and Licensure: Diplomate, National Board of Medical Examiners, 1975. Board Certified, American Board of Internal Medicine, 1978. Board Certified, Rheumatology subspecialty, 1982. California License: #G-30533. Present Appointments: Medical Director, Wallace Rheumatic Study Center Attune Health Affiliate, Beverly Hills, CA 90211 Attending Physician, Cedars-Sinai Medical Center, Los Angeles, 1979- Clinical Professor of Medicine, David Geffen School of Medicine at UCLA, 1995- Professor of Medicine, Cedars-Sinai Medical Center, 2012- Expert Reviewer, Medical Board of California, 2007- Associate Director, Rheumatology Fellowship Program, Cedars-Sinai Medical Center, 2010- Board of Governors, Cedars-Sinai Medical Center, 2016- Member, Medical Policy Committee, United Rheumatology, 2017- Honorary Appointments: Fellow, American College of Physicians (FACP) Fellow, American College of Rheumatology (FACR) -

New Biological Therapies: Introduction to the Basis of the Risk of Infection
New biological therapies: introduction to the basis of the risk of infection Mario FERNÁNDEZ RUIZ, MD, PhD Unit of Infectious Diseases Hospital Universitario “12 de Octubre”, Madrid ESCMIDInstituto de Investigación eLibraryHospital “12 de Octubre” (i+12) © by author Transparency Declaration Over the last 24 months I have received honoraria for talks on behalf of • Astellas Pharma • Gillead Sciences • Roche • Sanofi • Qiagen Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Paul Ehrlich (1854-1915) • “side-chain” theory (1897) • receptor-ligand concept (1900) • “magic bullet” theory • foundation for specific chemotherapy (1906) • Nobel Prize in Physiology and Medicine (1908) (together with Metchnikoff) Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author 1981: B-1 antibody (tositumomab) anti-CD20 monoclonal antibody 1997: FDA approval of rituximab for the treatment of relapsed or refractory CD20-positive NHL 2001: FDA approval of imatinib for the treatment of chronic myelogenous leukemia Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Functional classification of targeted (biological) agents • Agents targeting soluble immune effector molecules • Agents targeting cell surface receptors -

Statistical Analysis Plan
Official Title: A Phase IIIb, Open-Label Study to Evaluate the Safety and Tolerability of Shorter Infusions of Ocrelizumab in Patients With Primary Progressive and Relapsing Multiple Sclerosis NCT Number: NCT03606460 Document Date: SAP Version 1: 26-June-2019 STATISTICAL ANALYSIS PLAN TITLE: A PHASE IIIB, OPEN-LABEL STUDY TO EVALUATE THE SAFETY AND TOLERABILITY OF SHORTER INFUSIONS OF OCRELIZUMAB IN PATIENTS WITH PRIMARY PROGRESSIVE AND RELAPSING MULTIPLE SCLEROSIS PROTOCOL NUMBER: ML40638 STUDY DRUG: Ocrelizumab (RO4964913) VERSION NUMBER: 1 IND NUMBER: 100,593 EUDRACT NUMBER: Not applicable SPONSOR: Genentech, Inc. PLAN PREPARED BY: DATE FINAL: 26 June, 2019 STATISTICAL ANALYSIS PLAN APPROVAL Approved by Ph.D. on June 26, 2019 CONFIDENTIAL This is a Genentech, Inc. document that contains confidential information. Nothing herein is to be disclosed without written consent from Genentech, Inc. Ocrelizumab—Genentech, Inc. Statistical Analysis Plan ML40638 Clinical Study Report: Ocrelizumab — Genentech, Inc. CSR ML40638 370 TABLE OF CONTENTS 1. BACKGROUND ............................................................................................ 5 2. STUDY DESIGN ........................................................................................... 5 2.1 Protocol Synopsis .................................................................... 6 2.2 Outcome Measures ................................................................. 6 2.2.1 Primary Endpoint ..................................................................... 6 2.2.2 Secondary -

The Ocrelizumab Pharmacy Service at the Leeds Teaching Hospitals NHS Trust (LTHT): Improving the Patient Experience
The Ocrelizumab Pharmacy Service at the Leeds Teaching Hospitals NHS Trust (LTHT): Improving the Patient Experience Jeremy Robson & Sumrah Shaffiq, Leeds Teaching Hospitals NHS Trust Neurology Academy MS Advanced MasterClass 9.2 Background In 2018, the approval of Ocrevus (ocrelizumab) offered another treatment option for the management of relapsing remitting Multiple Sclerosis (RRMS)1, but the breakthrough decision came in 2019 upon its approval for early primary progressive MS (PPMS)2. The West Yorkshire MS Treatment Programme (WYMST) was set up to centralise a multi- district clinic and provides an effective model to ensure appropriate and equitable treatment for people with MS3. The WYMST has approximately 600 RRMS and approximately 100 PPMS from Leeds. Patients eligible for ocrelizumab are consented by the MS team and the prescribing is undertaken by independent pharmacist prescribers (IPPs). Ocrelizumab is administered on the LTHT day case unit, which is also used for the management of other neurology conditions. The IPPs contribute to the prescribing for these patients. Prescribing of ocrelizumab is undertaken on paper drug charts. At LTHT, the compounding of a majority of monoclonal antibodies (MAbs) (e.g. rituximab, alemtuzumab, infliximab, ocrelizumab) are overseen by the aseptics department. This is the default position to guarantee a sterile product and also mitigate the potential risk to staff members. This part of the service review involved challenging discussions between the IPPs, their aseptics colleagues and the MS team. The approval of ocrelizumab for RRMS and PPMS means a potential increase in prescribing, day case admissions and aseptics involvement in the manufacture of MAbs. A service review was warranted to establish if the current approach ensured the LTHT ocrelizumab service was efficient, safe and patient-centred. -

Attachment: Extract from Clinical Evaluation Ocrelizumab
AusPAR Attachment 2 Extract from the Clinical Evaluation Report for ocrelizumab Proprietary Product Name: Ocrevus Sponsor: Roche Products Pty Limited First round report: October 2016 Second round report: February 2017 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) · The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health, and is responsible for regulating medicines and medical devices. · The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance), when necessary. · The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. · The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. · To report a problem with a medicine or medical device, please see the information on the TGA website < https://www.tga.gov.au>. About the Extract from the Clinical Evaluation Report · This document provides a more detailed evaluation of the clinical findings, extracted from the Clinical Evaluation Report (CER) prepared by the TGA. This extract does not include sections from the CER regarding product documentation or post market activities. · The words (Information redacted), where they appear in this document, indicate that confidential information has been deleted. · For the most recent Product Information (PI), please refer to the TGA website < https://www.tga.gov.au/product-information-pi>. -
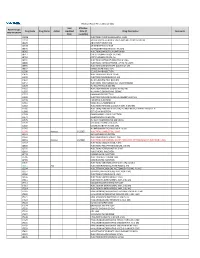
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -

SUPPLEMENTARY APPENDIX 4: Search Strategies Syntax Guide For
SUPPLEMENTARY APPENDIX 4: Search Strategies Pubmed, Embase Perioperative Management - PubMed Search Strategy – March 6, 2016 Syntax Guide for PubMed [MH] = Medical Subject Heading, also [TW] = Includes all words and numbers in known as MeSH the title, abstract, other abstract, MeSH terms, MeSH Subheadings, Publication Types, Substance Names, Personal Name as Subject, Corporate Author, Secondary Source, Comment/Correction Notes, and Other Terms - typically non-MeSH subject terms (keywords)…assigned by an organization other than NLM [SH] = a Medical Subject Heading [TIAB] = Includes words in the title and subheading, e.g. drug therapy abstracts [MH:NOEXP] = a command to retrieve the results of the Medical Subject Heading specified, but not narrower Medical Subject Heading terms Boolean Operators OR = retrieves results that include at least AND = retrieves results that include all the one of the search terms search terms NOT = excludes the retrieval of terms from the search Perioperative Management PubMed Search Strategy – March 6, 2016 Search Query #1 ((((ARTHROPLASTY, REPLACEMENT, HIP[MH] OR HIP PROSTHES*[TW] OR HIP REPLACEMENT*[TIAB] OR HIP ARTHROPLAST*[TIAB] OR HIP TOTAL REPLACEMENT*[TIAB] OR FEMORAL HEAD PROSTHES*[TIAB]) OR (ARTHROPLASTY, REPLACEMENT, KNEE[MH] OR KNEE PROSTHES*[TW] OR KNEE REPLACEMENT*[TW] OR KNEE ARTHROPLAST*[TW] OR KNEE TOTAL REPLACEMENT*[TIAB]) OR (ARTHROPLAST*[TW] AND (HIP[TIAB] OR HIPS[TIAB] OR KNEE*[TIAB])) AND (("1980/01/01"[PDAT] : "2016/03/06"[PDAT]) AND ENGLISH[LANG])) NOT (((("ADOLESCENT"[MESH]) OR "CHILD"[MESH]) -

Study Protocol
NCT0239061 Janssen Research & Development* Clinical Protocol A Multicenter, Randomized, Double-blind, Placebo-controlled, Proof-of-Concept Study of Ustekinumab in Subjects With Active Systemic Lupus Erythematosus CNTO1275SLE2001; Phase 2a AMENDMENT 3 STELARA® (ustekinumab) *Janssen Research & Development is a global organization that operates through different legal entities in various countries. Therefore, the legal entity acting as the Sponsor for Janssen Research & Development studies may vary, such as, but not limited to Janssen Biotech, Inc.; Janssen Products, LP; Janssen Biologics, BV; Janssen-Cilag International NV; Janssen, Inc; Janssen Infectious Diseases BVBA; Janssen R&D Ireland; or Janssen Research & Development, LLC. The term “Sponsor” is used throughout the protocol to represent these various legal entities; the Sponsor is identified on the Contact Information page that accompanies the protocol. This study will be conducted under US Food & Drug Administration IND regulations (21 CFR Part 312). EudraCT NUMBER: 2014-005000-19 Status: Approved Date: 18 January 2017 Prepared by: Janssen Research & Development, LLC EDMS number: EDMS-ERI-89608352, 10.0 GCP Compliance: This study will be conducted in compliance with Good Clinical Practice, and applicable regulatory requirements. Confidentiality Statement The information in this document contains trade secrets and commercial information that are privileged or confidential and may not be disclosed unless such disclosure is required by applicable law or regulations. In any event, persons to whom the information is disclosed must be informed that the information is privileged or confidential and may not be further disclosed by them. These restrictions on disclosure will apply equally to all future information supplied to you that is indicated as privileged or confidential. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell.