Transcript Profiling of a Novel Plant Meristem, the Monocot Cambium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Plant Terminology
PLANT TERMINOLOGY Plant terminology for the identification of plants is a necessary evil in order to be more exact, to cut down on lengthy descriptions, and of course to use the more professional texts. I have tried to keep the terminology in the database fairly simple but there is no choice in using many descriptive terms. The following slides deal with the most commonly used terms (more specialized terms are given in family descriptions where needed). Professional texts vary from fairly friendly to down-right difficult in their use of terminology. Do not be dismayed if a plant or plant part does not seem to fit any given term, or that some terms seem to be vague or have more than one definition – that’s life. In addition this subject has deep historical roots and plant terminology has evolved with the science although some authors have not. There are many texts that define and illustrate plant terminology – I use Plant Identification Terminology, An illustrated Glossary by Harris and Harris (see CREDITS) and others. Most plant books have at least some terms defined. To really begin to appreciate the diversity of plants, a good text on plant systematics or Classification is a necessity. PLANT TERMS - Typical Plant - Introduction [V. Max Brown] Plant Shoot System of Plant – stem, leaves and flowers. This is the photosynthetic part of the plant using CO2 (from the air) and light to produce food which is used by the plant and stored in the Root System. The shoot system is also the reproductive part of the plant forming flowers (highly modified leaves); however some plants also have forms of asexual reproduction The stem is composed of Nodes (points of origin for leaves and branches) and Internodes Root System of Plant – supports the plant, stores food and uptakes water and minerals used in the shoot System PLANT TERMS - Typical Perfect Flower [V. -
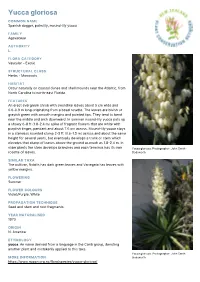
Yucca Gloriosa
Yucca gloriosa COMMON NAME Spanish dagger, palm lily, mound-lily yucca FAMILY Agavaceae AUTHORITY L. FLORA CATEGORY Vascular – Exotic STRUCTURAL CLASS Herbs - Monocots HABITAT Occur naturally on coastal dunes and shell mounds near the Atlantic, from North Carolina to north-east Florida. FEATURES An erect evergreen shrub with swordlike leaves about 5 cm wide and 0.6-0.9 m long originating from a basal rosette. The leaves are bluish or grayish green with smooth margins and pointed tips. They tend to bend near the middle and arch downward. In summer mound-lily yucca puts up a showy 6-8 ft (1.8-2.4 m) spike of fragrant flowers that are white with purplish tinges, pendant and about 7.6 cm across. Mound-lily yucca stays in a stemless rounded clump 2-5 ft (0.6-1.5 m) across and about the same height for several years, but eventually develops a trunk or stem which elevates that clump of leaves above the ground as much as 1.8-2.4 m. In older plants the stem develops branches and each terminus has its own Yucca gloriosa. Photographer: John Smith- rosette of leaves. Dodsworth SIMILAR TAXA The cultivar, Nobilis has dark green leaves and Variegata has leaves with yellow margins. FLOWERING Summer FLOWER COLOURS Violet/Purple, White PROPAGATION TECHNIQUE Seed and stem and root fragments. YEAR NATURALISED 1970 ORIGIN N. America ETYMOLOGY yucca: An name derived from a language in the Carib group, denoting another plant and mistakenly applied to this taxa. Yucca gloriosa. Photographer: John Smith- MORE INFORMATION Dodsworth https://www.nzpcn.org.nz/flora/species/yucca-gloriosa/. -

Tansley Review Evolution of Development of Vascular Cambia and Secondary Growth
New Phytologist Review Tansley review Evolution of development of vascular cambia and secondary growth Author for correspondence: Rachel Spicer1 and Andrew Groover2 Andrew Groover 1The Rowland Institute at Harvard, Cambridge, MA, USA; 2Institute of Forest Genetics, Pacific Tel: +1 530 759 1738 Email: [email protected] Southwest Research Station, USDA Forest Service, Davis, CA, USA Received: 29 December 2009 Accepted: 14 February 2010 Contents Summary 577 V. Evolution of development approaches for the study 587 of secondary vascular growth I. Introduction 577 VI. Conclusions 589 II. Generalized function of vascular cambia and their 578 developmental and evolutionary origins Acknowledgements 589 III. Variation in secondary vascular growth in angiosperms 581 References 589 IV. Genes and mechanisms regulating secondary vascular 584 growth and their evolutionary origins Summary New Phytologist (2010) 186: 577–592 Secondary growth from vascular cambia results in radial, woody growth of stems. doi: 10.1111/j.1469-8137.2010.03236.x The innovation of secondary vascular development during plant evolution allowed the production of novel plant forms ranging from massive forest trees to flexible, Key words: forest trees, genomics, Populus, woody lianas. We present examples of the extensive phylogenetic variation in sec- wood anatomy, wood formation. ondary vascular growth and discuss current knowledge of genes that regulate the development of vascular cambia and woody tissues. From these foundations, we propose strategies for genomics-based research in the evolution of development, which is a next logical step in the study of secondary growth. I. Introduction this pattern characterizes most extant forest trees, significant variation exists among taxa, ranging from extinct woody Secondary vascular growth provides a means of radially lycopods and horsetails with unifacial cambia (Cichan & thickening and strengthening plant axes initiated during Taylor, 1990; Willis & McElwain, 2002), to angiosperms primary, or apical growth. -

Are Cabbage Trees Worth Anything? Relating Ecological and Human Values in the Cabbage Tree, Fl Kouka
Are cabbage trees worth anything? Relating ecological and human values in the cabbage tree, fl kouka PHILIP SIMPSON piles, and fence posts. The cabbage tree exemplifies the relationship be Cabbage trees entered 'New Zealand' along tropical tween people and plants, not only the whole.tree itself archipelagos some 15 million years ago.' They have but also its parts, the leaves, the internal tissues, and adapted to the vicissitudes of sea-level, mountain build their biochemistry. ing and erosion, and climate change, and have evolved When Polynesian settlers named the New Zealand into different species and regional forms. A remarkable cabbage trees 'ti' they were retaining a tradition of set of structural and physiological features has devel using its tropical relative, Cordyline fruticosa (formerly oped to equip them for survival in a changeable and known as C. terminalis). They brought it with them and sometimes extreme environment. called it tl pore, 'the 'short' cabbage tree. European It was the qualities of strength and durability that whalers and sealers adopted 'cabbage palm' from Cook's were seized on by Maori, and they favoured some or first voyage because the tips of palms were often eaten selected new forms to satisfy their daily needs. The as a vegetable ('cabbage') by sailors, and because of the same qualities of survival underscore Pakeha respect archetypal similarity between cabbage trees and tropi for cabbage trees. They too have recognised variety and cal palms that the temperate travellers had seen for the have bred new forms to satisfy their aesthetic mores. A first time. It is perhaps the leafy, tropical look com sense of national identity has grown around the cab bined with temperate strength that account for the world bage tree and people have been shocked by the epi fame of cabbage trees. -

Mechanical Stress in the Inner Bark of 15 Tropical Tree Species and The
Mechanical stress in the inner bark of 15 tropical tree species and the relationship with anatomical structure Romain Lehnebach, Léopold Doumerc, Bruno Clair, Tancrède Alméras To cite this version: Romain Lehnebach, Léopold Doumerc, Bruno Clair, Tancrède Alméras. Mechanical stress in the inner bark of 15 tropical tree species and the relationship with anatomical structure. Botany / Botanique, NRC Research Press, 2019, 10.1139/cjb-2018-0224. hal-02368075 HAL Id: hal-02368075 https://hal.archives-ouvertes.fr/hal-02368075 Submitted on 18 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Mechanical stress in the inner bark of 15 tropical tree species and the relationship with anatomical structure1 Romain Lehnebach, Léopold Doumerc, Bruno Clair, and Tancrède Alméras Abstract: Recent studies have shown that the inner bark is implicated in the postural control of inclined tree stems through the interaction between wood radial growth and tangential expansion of a trellis fiber network in bark. Assessing the taxonomic extent of this mechanism requires a screening of the diversity in bark anatomy and mechanical stress. The mechanical state of bark was measured in 15 tropical tree species from various botanical families on vertical mature trees, and related to the anatomical structure of the bark. -

Plant Charts for Native to the West Booklet
26 Pohutukawa • Oi exposed coastal ecosystem KEY ♥ Nurse plant ■ Main component ✤ rare ✖ toxic to toddlers coastal sites For restoration, in this habitat: ••• plant liberally •• plant generally • plant sparingly Recommended planting sites Back Boggy Escarp- Sharp Steep Valley Broad Gentle Alluvial Dunes Area ment Ridge Slope Bottom Ridge Slope Flat/Tce Medium trees Beilschmiedia tarairi taraire ✤ ■ •• Corynocarpus laevigatus karaka ✖■ •••• Kunzea ericoides kanuka ♥■ •• ••• ••• ••• ••• ••• ••• Metrosideros excelsa pohutukawa ♥■ ••••• • •• •• Small trees, large shrubs Coprosma lucida shining karamu ♥ ■ •• ••• ••• •• •• Coprosma macrocarpa coastal karamu ♥ ■ •• •• •• •••• Coprosma robusta karamu ♥ ■ •••••• Cordyline australis ti kouka, cabbage tree ♥ ■ • •• •• • •• •••• Dodonaea viscosa akeake ■ •••• Entelea arborescens whau ♥ ■ ••••• Geniostoma rupestre hangehange ♥■ •• • •• •• •• •• •• Leptospermum scoparium manuka ♥■ •• •• • ••• ••• ••• ••• ••• ••• Leucopogon fasciculatus mingimingi • •• ••• ••• • •• •• • Macropiper excelsum kawakawa ♥■ •••• •••• ••• Melicope ternata wharangi ■ •••••• Melicytus ramiflorus mahoe • ••• •• • •• ••• Myoporum laetum ngaio ✖ ■ •••••• Olearia furfuracea akepiro • ••• ••• •• •• Pittosporum crassifolium karo ■ •• •••• ••• Pittosporum ellipticum •• •• Pseudopanax lessonii houpara ■ ecosystem one •••••• Rhopalostylis sapida nikau ■ • •• • •• Sophora fulvida west coast kowhai ✖■ •• •• Shrubs and flax-like plants Coprosma crassifolia stiff-stemmed coprosma ♥■ •• ••••• Coprosma repens taupata ♥ ■ •• •••• •• -

Non-Destructive Estimation of Root Pressure Using Sap Flow, Stem
Annals of Botany 111: 271–282, 2013 doi:10.1093/aob/mcs249, available online at www.aob.oxfordjournals.org Non-destructive estimation of root pressure using sap flow, stem diameter measurements and mechanistic modelling Tom De Swaef*, Jochen Hanssens, Annelies Cornelis and Kathy Steppe Faculty of Bioscience Engineering, Department of Applied Ecology and Environmental Biology, Laboratory of Plant Ecology, Ghent University, Coupure links 653, B-9000 Ghent, Belgium * For correspondence. E-mail [email protected] Received: 20 August 2012 Returned for revision: 24 September 2012 Accepted: 8 October 2012 Published electronically: 4 December 2012 † Background Upward water movement in plants via the xylem is generally attributed to the cohesion–tension theory, as a response to transpiration. Under certain environmental conditions, root pressure can also contribute Downloaded from to upward xylem water flow. Although the occurrence of root pressure is widely recognized, ambiguity exists about the exact mechanism behind root pressure, the main influencing factors and the consequences of root pres- sure. In horticultural crops, such as tomato (Solanum lycopersicum), root pressure is thought to cause cells to burst, and to have an important impact on the marketable yield. Despite the challenges of root pressure research, progress in this area is limited, probably because of difficulties with direct measurement of root pressure, prompt- ing the need for indirect and non-destructive measurement techniques. http://aob.oxfordjournals.org/ † Methods A new approach to allow non-destructive and non-invasive estimation of root pressure is presented, using continuous measurements of sap flow and stem diameter variation in tomato combined with a mechanistic flow and storage model, based on cohesion–tension principles. -

Chapter 5: the Shoot System I: the Stem
Chapter 5 The Shoot System I: The Stem THE FUNCTIONS AND ORGANIZATION OF THE SHOOT SYSTEM PRIMARY GROWTH AND STEM ANATOMY Primary Tissues of Dicot Stems Develop from the Primary Meristems The Distribution of the Primary Vascular Bundles Depends on the Position of Leaves Primary Growth Differs in Monocot and Dicot Stems SECONDARY GROWTH AND THE ANATOMY OF WOOD Secondary Xylem and Phloem Develop from Vascular Cambium Wood Is Composed of Secondary Xylem Gymnosperm Wood Differs from Angiosperm Wood Bark Is Composed of Secondary Phloem and Periderm Buds Are Compressed Branches Waiting to Elongate Some Monocot Stems Have Secondary Growth STEM MODIFICATIONS FOR SPECIAL FUNCTIONS THE ECONOMIC VALUE OF WOODY STEMS SUMMARY ECONOMIC BOTANY: How Do You Make A Barrel? 1 KEY CONCEPTS 1. The shoot system is composed of the stem and its lateral appendages: leaves, buds, and flowers. Leaves are arranged in different patterns (phyllotaxis): alternate, opposite, whorled, and spiral. 2. Stems provide support to the leaves, buds, and flowers. They conduct water and nutrients and produce new cells in meristems (shoot apical meristem, primary and secondary meristems). 3. Dicot stems and monocot stems are usually different. Dicot stems tend to have vascular bundles distributed in a ring, whereas in monocot stems they tend to be scattered. 4. Stems are composed of the following: epidermis, cortex and pith, xylem and phloem, and periderm. 5. Secondary xylem is formed by the division of cells in the vascular cambium and is called wood. The bark is composed of all of the tissues outside the vascular cambium, including the periderm (formed from cork cambium) and the secondary phloem. -

Mechanical Properties of Blueberry Stems
Vol. 64, 2018 (4): 202–208 Res. Agr. Eng. https://doi.org/10.17221/90/2017-RAE Mechanical properties of blueberry stems Margus Arak1, Kaarel Soots1*, Marge Starast2, Jüri Olt1 1Institute of Technology, Estonian University of Life Sciences, Tartu, Estonia 2Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences, Tartu, Estonia *Corresponding author: [email protected] Abstract Arak M., Soots K., Starast M., Olt J. (2018): Mechanical properties of blueberry stems. Res. Agr. Eng., 64: 202–208. In order to model and optimise the structural parameters of the working parts of agricultural machines, including har- vesting machines, the mechanical properties of the culture harvested must be known. The purpose of this article is to determine the mechanical properties of the blueberry plant’s stem; more precisely the tensile strength and consequent elastic modulus E. In order to achieve this goal, the measuring instrument Instron 5969L2610 was used and accompany- ing software BlueHill 3 was used for analysing the test results. The tested blueberry plant’s stems were collected from the blueberry plantation of the Farm Marjasoo. The diameters of the stems were measured, test units were prepared, tensile tests were performed, tensile strength was determined and the elastic modulus was obtained. Average value of the elastic modulus of the blueberry (Northblue) plant’s stem remained in the range of 1268.27–1297.73 MPa. Keywords: agricultural engineering; blueberry; harvesting; mechanical properties; elastic modulus Lowbush blueberry (Vaccinium angustifolium abandoned peat fields because of soils with high Ait.) is a native naturally occurring plant in North organic matter content and low pH (Yakovlev et America. -

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -
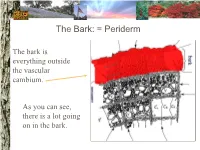
The Bark: = Periderm
The Bark: = Periderm The bark is everything outside the vascular cambium. As you can see, there is a lot going on in the bark. The Bark: periderm: phellogen (cork cambium): The phellogen is the region of cell division that forms the periderm tissues. Phellogen development influences bark appearance. The Bark: periderm: phellem (cork): Phellem replaces the epidermis as the tree increases in girth. Photosynthesis can take place in some trees both through the phellem and in fissures. The Bark: periderm: phelloderm: Phelloderm is active parenchyma tissue. Parenchyma cells can be used for storage, photosynthesis, defense, and even cell division! The Bark: phloem: Phloem tissue makes up the inner bark. However, it is vascular tissue formed from the vascular cambium. The Bark: phloem: sieve tube elements: Sieve tube elements actively transport photosynthates down the stem. Conifers have sieve cells instead. The cambium: The cambium is the primary meristem producing radial growth. It forms the phloem & xylem. The Xylem (wood): The xylem includes everything inside the vascular cambium. The Xylem: a growth increment (ring): The rings seen in many trees represent one growth increment. Growth rings provide the texture seen in wood. The Xylem: vessel elements: Hardwood species have vessel elements in addition to trachieds. Notice their location in the growth rings of this tree The Xylem: fibers: Fibers are cells with heavily lignified walls making them stiff. Many fibers in sapwood are alive at maturity and can be used for storage. The Xylem: axial parenchyma: Axial parenchyma is living tissue! Remember that parenchyma cells can be used for storage and cell division.