Quantum Mechanics and Particle Scattering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harry Moseley: Numbering the Elements
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 5. Harry Moseley: Numbering the Elements Dmitri Mendeleev (identified on screen) works on the Periodic Table, writes down the atomic weights of the elements. NARR: Previously on The Mystery of Matter… HISTORIAN MICHAEL GORDIN VO He figures out something rather extraordinary about the elements. DMITRI MENDELEEV, partly in VO The eye is immediately struck by a pattern within the horizontal rows and the vertical columns. Mendeleev’s first table morphs into the familiar modern Periodic Table. AUTHOR ERIC SCERRI VO He found an absolutely fundamental principle of nature. Humphry Davy (identified on screen) performs an experiment with his voltaic pile. HISTORIAN DAVID KNIGHT VO Somehow the particles of matter have to be glued together to form molecules. What Davy has had is a big idea. Perhaps electricity could be this kind of glue. Marie Curie (identified on screen) sits down at the spectroscope and peers into the eyepiece. NARR: The spectroscope kicked off a whole new round in the discovery of elements. The spectra of four elements appear on screen, along with their names. PHYSICIST DAVID KAISER VO It’s almost like each element has its own bar code. Marie and Pierre enter their lab at night and see vials of radium glowing on the shelves. MARIE TO PIERRE Don’t light the lamps! Look! 1 MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS PHYSICIST DAVID KAISER VO Radioactivity was a sign that the atom itself was unstable. It could break apart. Marie and Pierre look in wonder at their radiant element. NARR: Scientists now had a pressing new question to answer: What’s inside the atom? Fade to black ANNOUNCER: Major funding for The Mystery of Matter: Search for the Elements was provided by the National Science Foundation, where discoveries begin. -

Radiation and Radioactivity Quantified? Do You Think of These “People” When I Say RADIATION? Do You Think of These Things As Well?
Welcome To RadTown USA •Click to Explore RadTown USA • Click on any location in RadTown USA and find out about radiation sources or uses at that location. The Alpha, Beta, Gammas of Nuclear Education March 2nd, 2014 Fundamentals of Ionizing Radiation Debra N Thrall, PhD Executive Director Albert I Pierce Foundation Radiation Fundamentals What is radiation? Where does it come from? How does it interact with matter? What is radioactivity? What are fission and fusion? How are radiation and radioactivity quantified? Do you think of these “people” when I say RADIATION? Do you think of these things as well? • Food • Space • Utilities • Consumer Products • Medicine Brief History of the Atom • 500 BC Democritus Atom • Long time (Romans Dark Ages) • 1808 AD Dalton Plum Pudding • 1911 Rutherford Nucleus • 1913 Bohr Orbits • 1920’s Many People Quantum Mechanics Rutherford’s Gold Foil Experiment The Design 1. Bombard positively charged alpha particles into thin gold foil. 2. Use fluorescent screen to detect particles as they exit the gold foil. 3. Use angle of deflection to determine interior of the atom. So, What is an Atom? • Atoms are made up of protons, neutrons & electrons • Protons: + charge p+ • Neutrons: no charge n0 • Electrons: - charge e- • Atoms want to have a stable energy level • This translates to having no net charge • # protons = # electrons Mass of an Atom • Masses • Proton: 1.000000 amu • Neutron: 1.000000 amu • Electron: 0.000549 amu (Translates to 1.2 lbs/1 ton ~ a kitten on an elephant!) • The mass of an atom is approximately -
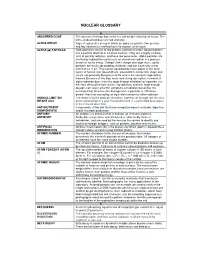
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Dosimetry of Low-Energy Beta Radiation
Risø-R-907(EN) Dosimetiy of Low-Energy Beta Radiation Jette Borg Risø National Laboratory, Roskilde, Denmark August 1996 VOL 2 7 »22 Dosimetry of Low-Energy Beta Radiation Jette Borg Risø National Laboratory, Roskilde, Denmark August 1996 Abstract Useful techniques and procedures for determination of absorbed doses from exposure in a low-energy /? radiation field were studied and evaluated in this project. The four different techniques included were /? spectrometry, extrapolation chamber dosimetry, Monte Carlo (MC) calculations, and exoelectron dosimetry. As a typical low-energy /? radiation field a moderated spectrum from a 14C source {Ep^max — 156 keV) was chosen for the study. The measured response of a Si(Li) detector to photons (bremsstrahlung) showed fine agreement with the MC calculated photon response, whereas the difference be- tween measured and MC calculated responses to electrons indicates an additional deadlayer thickness of about 12 /xm in the Si(Li) detector. The depth-dose profiles measured with extrapolation chambers at two labora- tories agreed very well, and it was confirmed that the fitting procedure previous- ly reported for 147Pm depth-dose profiles is also suitable for /? radiation from 14C. An increasing difference between measured and MC calculated dose rates for increasing absorber thicknesses was found, which is explained by limitations of the EGS4 code for transport of very low energy electrons (below 10 - 20 keV). Finally, a study of the thermally stimulated exoelectron emission (TSEE) re- sponse of BeO thin film dosemeters to /? radiation for radiation fields with maxi- mum P energies ranging from 67 keV to 2.27 MeV is reported. -

Rutherford Scattering
Rutherford Scattering MIT Department of Physics (Dated: September 24, 2014) This is an experiment which studies scattering alpha particles on atomic nuclei. You will shoot alpha particles, emitted by 241Am, at thin metal foils and measure the scattering cross section of the target atoms as a function of the scattering angle, the alpha particle energy, and the nuclear charge. You will then measure the intensity of alpha particles scattered by thin metal foils as a function of the scattering angle for several elements of very different atomic number. PREPARATORY QUESTIONS within which the electrons occupy certain positions of equilibrium, like raisins in a pudding. Set in motion, Please visit the Rutherford Scattering chapter on the the electrons should vibrate harmonically, radiating elec- 8.13x website at mitx.mit.edu to review the background tromagnetic energy with characteristic sharp frequencies material for this experiment. Answer all questions found that would be in the optical range if the radii of the −8 in the chapter. Work out the solutions in your laboratory atomic spheres were of the order of 10 cm. However, notebook; submit your answers on the web site. the \raisin pudding" model yielded no explanation of the numerical regularities of optical spectra, e.g. the Balmer formula for the hydrogen spectrum and the Ritz combi- SUGGESTED PROGRESS CHECK FOR END OF nation principle [3] for spectra in general. 2nd SESSION At this point, Ernest Rutherford got the idea that the structure of atoms could be probed by observing Plot the rate of alpha particle observations for 10◦ and the scattering of alpha particles. -

Development of a 2K Joule-Thomson Closed-Cycle Cryocooler
P# 1 81 Development of a 2K Joule-Thomson Closed-Cycle Cryocooler M. Crook, T. Bradshaw, G. Gilley, M. Hills, S. Watson, B. Green, C. Pulker, T. Rawlings STFC Rutherford Appleton Laboratory, Harwell, Oxford OX11 0QX ABSTRACT The Rutherford Appleton Laboratory (RAL) is currently developing a 2K Joule-Thomson cooler targeted at future space science missions requiring low temperatures, for example as part of the cryogenic chain for the ESA Athena X-ray telescope. The cooler builds on previous closed cycle cooler developments at RAL, in particular the very successful 4K-JT cooler that was flown on the ESA Planck mission. However the working fluid for the 2K-JT cooler will be 3He, and two extra stages of compression have been added to provide a much lower effluent pressure and a greater overall compression ratio. The design of the 2K-JT cooler is described, with reference to lessons learnt from Planck in-orbit data, and results of the test program to date are presented. INTRODUCTION Cryocooler development for space applications has been ongoing for over 30 years at RAL1, and has resulted in a range of closed cycle mechanical cryocoolers covering temperatures from 2 K to 80 K and above. These coolers have been licensed to both UK and US industry and have subsequently under- pinned a large variety of high profile scientific and operational missions; ranging from space exploration and the origins of the universe, to earth observation, climate change, and weather forecasting. One of those developments was a 4K Joule-Thomson (4K-JT) cooler2 which was flown on the ESA Planck mission3 to observe the fluctuations of the Cosmic Microwave Background. -

PHYS 5012 Radiation Physics and Dosimetry
Radiation Physics Lecture 1 Background and Fundamentals The Discovery of Radiation X-rays Radioactivity Classification of Radiation Types of Ionising Radiation PHYS 5012 Radiation Units and Properties Radiation Physics and Dosimetry Dose in Water Atomic Physics and Radiation Lecture 1 The Rutherford-Bohr Model Multi-Electron Atoms Production of Radiation Characteristic Radiation Characteristic X-rays Auger Electrons Continuous Radiation Bremsstrahlung Radiation Tuesday 5 March 2013 Synchrotron Radiation Cerenkov Radiation Particle Accelerators X-ray Tubes Cyclotrons Linear Accelerators Radiation Physics Lecture 1 The Discovery of Radiation Background and Fundamentals The Discovery of Radiation X-rays Radioactivity Classification of Radiation Types of Ionising Radiation Three main discoveries of radiation made at the turn of Radiation Units and Properties the 19th century, together with several major advances in Dose in Water Atomic Physics and theoretical physics, including quantum mechanics and Radiation The Rutherford-Bohr special relativity, signalled the birth of Radiation Physics. Model Multi-Electron Atoms The subsequent realisation that radiation can be harmful Production of Radiation to humans led to the the rapid development of radiation Characteristic Radiation Characteristic X-rays dosage measurements and quantification and commonly Auger Electrons accepted standards for tolerable levels of radiation in Continuous Radiation Bremsstrahlung Radiation humans. Synchrotron Radiation Cerenkov Radiation Particle Accelerators X-ray Tubes -

P4 ATOMIC STRUCTURE Question Practice Class: ______Date: ______
Name: ________________________ P4 ATOMIC STRUCTURE Question Practice Class: ________________________ Date: ________________________ Time: 132 minutes Marks: 129 marks Comments: HIGHER TIER Page 1 of 44 (a) The graph shows how the count rate from a sample containing the radioactive substance 1 cobalt-60 changes with time. (i) What is the range of the count rate shown on the graph? From __________ counts per second to __________ counts per second. (1) (ii) How many years does it take for the count rate to fall from 200 counts per second to 100 counts per second? Time = _________________________ years (1) (iii) What is the half-life of cobalt-60? Half-life = _________________________ years (1) Page 2 of 44 (b) The gamma radiation emitted from a source of cobalt-60 can be used to kill the bacteria on fresh, cooked and frozen foods. Killing the bacteria reduces the risk of food poisoning. The diagram shows how a conveyor belt can be used to move food past a cobalt-60 source. (i) Which one of the following gives a way of increasing the amount of gamma radiation the food receives? Put a tick ( ) in the box next to your answer. Increase the temperature of the cobalt-60 source. Make the conveyor belt move more slowly. Move the cobalt-60 source away from the conveyor belt. (1) (ii) To protect people from the harmful effects of the gamma radiation, the cobalt-60 source has thick metal shielding. Which one of the following metals should be used? Draw a ring around your answer. aluminium copper lead (1) Page 3 of 44 (c) A scientist has compared the vitamin content of food exposed to gamma radiation with food that has not been exposed. -

RUT - Rutherford Scattering Physics 111B: Advanced Experimentation Laboratory University of California, Berkeley
RUT - Rutherford Scattering Physics 111B: Advanced Experimentation Laboratory University of California, Berkeley Contents 1 Rutherford Scattering Description (RUT)1 2 Rutherford Experiment Pictures2 3 Before the 1st Day of Lab and SOP for this experiment2 4 Objectives 3 5 Introduction 4 6 Equipment used in this experiment4 7 Theory 5 8 Experimental Overview 6 9 Procedure: Getting Started on Rutherford Scattering7 10 Electronics 8 11 PHA Setup and Operation9 12 Handling Gold Foils 9 13 Data Collection (see Appendix on Error Analysis)9 14 Questions 10 15 References 10 1 Rutherford Scattering Description (RUT) 1. Note that there is NO eating or drinking in the 111-Lab anywhere, except in rooms 282 & 286 LeConte on the bench with the BLUE stripe around it. Thank you { the Staff. An experiment that shook the world of physics was carried out by Rutherford in 1910. He found that when scattering alpha particles from gold foils, far more of them were scattered at large angles than were expected to by theory. He explained the finding by assuming that all of the mass and positive charge of an atom are concentrated in a small volume at the center, in a compact nucleus. He derived an equation in which the differential cross section for scattering by an atomic nucleus is proportional to the inverse fourth power of the sine of the scattering angle. In this experiment the scattering formula is confirmed for the case of alpha particles scattered by gold nuclei in a thin gold foil. The charge on the gold nucleus will also be determined. -

Radiation F12
Radiation / Radioactivity / Radioactive Decay Radioactive Particles / Common Isotopes Counting History – Discovery of X-rays / Radioactivity / Nuclear atom Radioactive Decay – particles, half-life and equations Radioactivity – the Nuclear atom / trip to the “Particle Zoo” Nuclear Energy - fission and fusion Common Radio isotopes / C Isotopes – C-12 / C-13 / C-14 C-14 and radiocarbon dating Counting: Film / Geiger Counter / LSC / PI Terms: Radioactivity / Exposure / Dose Hackert – CH370 Sept. 1895 - Marconi (radio waves / wireless) Nov. 8, 1895 - Rontgen (discovery of X-rays) Feb. 24, 1896 – Becquerel (U luminesce”) (Feb. 26, 27 - cloudy days) (Mar. 1 - “radioactivity”) 1897 - JJ Thomson (discovery of electrons) 1898 – Pierre & Marie Curie (Po, Ra) 1898 – Rutherford (a and b radiation) 1902 – Rutherford (disintegration of elements) 1911 – Rutherford (Au foil exp. / nuclear atom) 1912 – von Laue (X-rays as waves) 1913 Braggs – 1st crystal structure 1920 – Rutherford (predicts neutron) Sept. 1895 - Marconi (radio waves / wireless) Nov. 8, 1895 - Rontgen (discovery of X-rays) Feb. 24, 1896 – Becquerel (U luminesce”) (Feb. 26, 27 - cloudy days) (Mar. 1 - “radioactivity”) 1897 - JJ Thomson (discovery of electrons) 1898 – Pierre & Marie Curie (Po, Ra) 1898 – Rutherford (a and b radiation) 1902 – Rutherford (disintegration of elements) 1911 – Rutherford (Au foil exp. / nuclear atom) 1912 – von Laue (X-rays as waves) 1913 Braggs – 1st crystal structure 1920 – Rutherford (predicts neutron) - l t Rutherford – quantitative I = Io e measurements Nuclear Wall Chart – Lawrence Berkeley Natl. Laboratory What is a “half-life”? A/A o = exp(-kt) So, when half of the sample is gone: A/A o = ½ or A o /A = 2 ln(2) = k * Half-life Half-life = ln(2)/k = 0.693/k Characteristics of Biologically Significant Isotopes - l t I = Io e iClicker Question #2: Radioactivity: Consider an isotope of “Te” with 77 neutrons that undergoes beta (+) decay with a half life of 22.4 days. -

Radiation-Hardness Testing of Electronic Devices: a Survey of Facility Dosimetry Practices
NBSIR 76-1135 Radiation-Hardness Testing of Electronic Devices: A Survey of Facility Dosimetry Practices J. C. Humphreys and S. E. Chappell Center for Radiation Research Institute for Basic Standards National Bureau of Standards Washington, D. C. 20234 September 1976 Final Prepared for Defense Nuclear Agency Washington, D. C. 20305 Sponsored Under: Subtask Code Z99QAXD072 and Work Unit 05 NBSIR 76-1135 RADIATION-HARDNESS TESTING OF ELECTRONIC DEVICES: A SURVEY OF FACILITY DOSIMETRY PRACTICES J. C. Humphreys and S. E. Chappell Center for Radiation Research Institute for Basic Standards National Bureau of Standards Washington, D. C. 20234 September 1976 Final Prepared for Defense Nuclear Agency Washington, D. C. 20305 Sponsored Under: Subtask Code Z99QAXD072 and Work Unit 05 U S. DEPARTMENT OF COMMERCE, Elliot L. Richardson, Secretary Edward O. Vetter. Under Secretary Dr. Betsy Ancker-Johnson, Assistant Secretary for Science and Technology NATIONAL BUREAU OF STANDARDS. Ernest Ambler. Acting Director Table of Contents Page ABSTRACT 5 1. INTRODUCTION 6 2. FACILITIES VISITED 6 3. RADIATION SOURCES 7 3.1 Isotope Sources 7 3.2 Flash Accelerators 10 3.3 Linacs and D.C. Accelerators 10 4. CALIBRATION OF THE RADIATION SOURCES 11 4.1 y-Ray Isotope Source Calibration ... 11 4.2 Accelerator Calibration 13 5. DOSIMETRY SYSTEMS EMPLOYED DURING DEVICE IRRADIATION 14 5.1 Thermoluminescence Dosimeters 14 5.2 Dosimeter Calibration 14 5.3 Dosimeter Readout Devices 16 5.4 Dosimeter Handling Procedures 17 5.5 Use of Dosimeters During Device Irradiation 19 6. COMMENTS FROM FACILITY PERSONNEL 19 7. OBSERVATIONS ON DOSIMETRY PRACTICES 20 8. RECOMMENDATIONS 21 REFERENCES 22 APPENDIX 23 3 List of Tables Page 1. -

Harnessed Atom: Nuclear Energy & Electricity Photosynthesis Biomass Is the Name for Materials from Plants and Animals That Have Chemical Energy Stored in Them
Table of Contents Lesson 1 - Energy Basics Lesson 2 - Electricity Basics Lesson 3 - Atoms and Isotopes Lesson 4 - Ionizing Radiation Lesson 5 - Fission, Chain Reactions Lesson 6 - Atom to Electricity Lesson 7 - Waste from Nuclear Power Plants Lesson 8 - Concerns Lesson 9 - Energy and You Lesson 1 ENERGY BASICS Radiant Chemical Energy Energy Mechanical Energy Introduction This lesson will look at the states and forms of energy. Next we will look at where energy comes from. Finally, we’ll explore how the way we live is tied to our energy supply and what that means for the future. TOPICS: States of energy What is energy? Potential Energy is the ability to do work. But We use energy all the time. Whenever Kinetic what does that really mean? work is done, energy is used. All activities Forms of energy Mechanical involve energy. Chemical You might think of work as cleaning your Nuclear Electrical room, cutting the grass, or studying for a We need energy to Radiant test. And all these require energy. • power our factories and businesses Energy from gravity • heat and light our homes and schools Thermal Energy sources To a scientist, “work” means something • run our appliances and machines Primary and secondary more exact. Work is causing a change. It • stay alive and keep our bodies moving sources Renewable vs non- can be a change in position, like standing • build and fuel our cars, trucks, planes, renewable up or moving clothes from the floor to and ships Conversion Conservation the laundry basket. It can be a change in • run television and videos Environmental effects temperature, like heating water for a cup • power our phones, computers, music, Greenhouse effect of tea.