Radiation and Radioactivity Quantified? Do You Think of These “People” When I Say RADIATION? Do You Think of These Things As Well?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harry Moseley: Numbering the Elements
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 5. Harry Moseley: Numbering the Elements Dmitri Mendeleev (identified on screen) works on the Periodic Table, writes down the atomic weights of the elements. NARR: Previously on The Mystery of Matter… HISTORIAN MICHAEL GORDIN VO He figures out something rather extraordinary about the elements. DMITRI MENDELEEV, partly in VO The eye is immediately struck by a pattern within the horizontal rows and the vertical columns. Mendeleev’s first table morphs into the familiar modern Periodic Table. AUTHOR ERIC SCERRI VO He found an absolutely fundamental principle of nature. Humphry Davy (identified on screen) performs an experiment with his voltaic pile. HISTORIAN DAVID KNIGHT VO Somehow the particles of matter have to be glued together to form molecules. What Davy has had is a big idea. Perhaps electricity could be this kind of glue. Marie Curie (identified on screen) sits down at the spectroscope and peers into the eyepiece. NARR: The spectroscope kicked off a whole new round in the discovery of elements. The spectra of four elements appear on screen, along with their names. PHYSICIST DAVID KAISER VO It’s almost like each element has its own bar code. Marie and Pierre enter their lab at night and see vials of radium glowing on the shelves. MARIE TO PIERRE Don’t light the lamps! Look! 1 MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS PHYSICIST DAVID KAISER VO Radioactivity was a sign that the atom itself was unstable. It could break apart. Marie and Pierre look in wonder at their radiant element. NARR: Scientists now had a pressing new question to answer: What’s inside the atom? Fade to black ANNOUNCER: Major funding for The Mystery of Matter: Search for the Elements was provided by the National Science Foundation, where discoveries begin. -

Civil Defense and Homeland Security: a Short History of National Preparedness Efforts
Civil Defense and Homeland Security: A Short History of National Preparedness Efforts September 2006 Homeland Security National Preparedness Task Force 1 Civil Defense and Homeland Security: A Short History of National Preparedness Efforts September 2006 Homeland Security National Preparedness Task Force 2 ABOUT THIS REPORT This report is the result of a requirement by the Director of the Department of Homeland Security’s National Preparedness Task Force to examine the history of national preparedness efforts in the United States. The report provides a concise and accessible historical overview of U.S. national preparedness efforts since World War I, identifying and analyzing key policy efforts, drivers of change, and lessons learned. While the report provides much critical information, it is not meant to be a substitute for more comprehensive historical and analytical treatments. It is hoped that the report will be an informative and useful resource for policymakers, those individuals interested in the history of what is today known as homeland security, and homeland security stakeholders responsible for the development and implementation of effective national preparedness policies and programs. 3 Introduction the Nation’s diverse communities, be carefully planned, capable of quickly providing From the air raid warning and plane spotting pertinent information to the populace about activities of the Office of Civil Defense in the imminent threats, and able to convey risk 1940s, to the Duck and Cover film strips and without creating unnecessary alarm. backyard shelters of the 1950s, to today’s all- hazards preparedness programs led by the The following narrative identifies some of the Department of Homeland Security, Federal key trends, drivers of change, and lessons strategies to enhance the nation’s learned in the history of U.S. -

Downloads of Technical Information
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2018 Nuclear Spaces: Simulations of Nuclear Warfare in Film, by the Numbers, and on the Atomic Battlefield Donald J. Kinney Follow this and additional works at the DigiNole: FSU's Digital Repository. For more information, please contact [email protected] FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES NUCLEAR SPACES: SIMULATIONS OF NUCLEAR WARFARE IN FILM, BY THE NUMBERS, AND ON THE ATOMIC BATTLEFIELD By DONALD J KINNEY A Dissertation submitted to the Department of History in partial fulfillment of the requirements for the degree of Doctor of Philosophy 2018 Donald J. Kinney defended this dissertation on October 15, 2018. The members of the supervisory committee were: Ronald E. Doel Professor Directing Dissertation Joseph R. Hellweg University Representative Jonathan A. Grant Committee Member Kristine C. Harper Committee Member Guenter Kurt Piehler Committee Member The Graduate School has verified and approved the above-named committee members, and certifies that the dissertation has been approved in accordance with university requirements. ii For Morgan, Nala, Sebastian, Eliza, John, James, and Annette, who all took their turns on watch as I worked. iii ACKNOWLEDGMENTS I would like to thank the members of my committee, Kris Harper, Jonathan Grant, Kurt Piehler, and Joseph Hellweg. I would especially like to thank Ron Doel, without whom none of this would have been possible. It has been a very long road since that afternoon in Powell's City of Books, but Ron made certain that I did not despair. Thank you. iv TABLE OF CONTENTS Abstract..............................................................................................................................................................vii 1. -

Duck and Cover: How Print Media, the U.S. Government, and Entertainment Culture Formedamerica's Understanding of the Atom
DUCK AND COVER: HOW PRINT MEDIA, THE U.S. GOVERNMENT, AND ENTERTAINMENT CULTURE FORMEDAMERICA’S UNDERSTANDING OF THE ATOM BOMB A thesis submitted in partial fulfillment of the requirements for the degree of Master of Arts By Daniel Patrick Wright B.A., University of Cincinnati, 2013 2015 Wright State University WRIGHT STATE UNIVERSITY GRADUATE SCHOOL May 5, 2015 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Daniel Patrick Wright ENTITLED Duck and Cover: How Print Media, the U.S. Government and Entertainment Culture Formed America’s Understanding of the Atom Bomb BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Arts ________________________________ Jonathan Winkler, Thesis Director ________________________________ Carol Herringer, Chair History Department Committee on College of Liberal Arts Final Examination ________________________________ Drew Swanson, Ph.D. ________________________________ Nancy Garner, Ph.D. ________________________________ Robert E. W. Fyffe, Ph.D. Vice President for Research and Dean of the Graduate School ABSTRACT Wright, Daniel Patrick. M.A. Department of History, Wright State University, 2015. Duck and Cover: How Print Media, the U.S. Government and Entertainment Culture Formed America’s Understanding of the Atom Bomb This research project will explore an overview of the different subsections of American post-war society that contributed to the American “atomic reality” in hopes of revealing how and why the American understanding of atomic weapons did not slowly evolve over the course of a generation, but instead materialize rapidly in the years following the bombing of Hiroshima and Nagasaki. By analyzing government sources and programs, print media sources such as newspapers and magazines, and the American entertainment culture of the 1940s and 1950s, this research project will answer exactly why and how the American public arrived at its understanding of the atom bomb. -

Planning for Emergencies and Disasters
Overview of New Castle County Floods Winter Storms Homeland Security Hazards and Disasters Know what to expect: Recommended Actions by Threat Level Family Preparations •Research the flood hazards in your area. New Castle County is vulnerable to a wide range of (www.fema.gov or www.nccde.org •Have heavy blankets on hand. GREEN=LOW natural hazards, includingincluding flooding, tornadoes, “Hurricane and Flood Safe”, link to tropical storms, hurricanes, winter storms, and •Ensure that each family member has a warm •Take steps to protect your identity. earthquakes. Their occurrence is natural and inevi- NFIP site) coat, gloves or mittens, and insulated •Report suspicious activity to the local police. table, and there is little we can do to control their water resistant boots. •If it has been raining hard for several hours, or (NCC Police: (302) 573-2800) or force and intensity. NCC is also vulnerable to a steadily for several days, be alert to the •Check on any elderly neighbors or family. 1-800-FORCE-12 Terrorism Tip Line variety of human-caused hazards, including chemi- possibility of a flood. (in locations such as cal releases, spills or explosions associated with small streams, creeks and roadways) •Consult a doctor regarding flu shots for •Take first aid and CPR classes. the fixed storage or mobile transport of hazardous family members. •Prepare a 72-hour family emergency supply kit materials. In today’s world, we must also consider •Listen to local radio and TV stations for flood •Prepare a 72-hour family emergency supply kit and plan. human-caused hazards, such as technological acci- information. -

Bob Farquhar
1 2 Created by Bob Farquhar For and dedicated to my grandchildren, their children, and all humanity. This is Copyright material 3 Table of Contents Preface 4 Conclusions 6 Gadget 8 Making Bombs Tick 15 ‘Little Boy’ 25 ‘Fat Man’ 40 Effectiveness 49 Death By Radiation 52 Crossroads 55 Atomic Bomb Targets 66 Acheson–Lilienthal Report & Baruch Plan 68 The Tests 71 Guinea Pigs 92 Atomic Animals 96 Downwinders 100 The H-Bomb 109 Nukes in Space 119 Going Underground 124 Leaks and Vents 132 Turning Swords Into Plowshares 135 Nuclear Detonations by Other Countries 147 Cessation of Testing 159 Building Bombs 161 Delivering Bombs 178 Strategic Bombers 181 Nuclear Capable Tactical Aircraft 188 Missiles and MIRV’s 193 Naval Delivery 211 Stand-Off & Cruise Missiles 219 U.S. Nuclear Arsenal 229 Enduring Stockpile 246 Nuclear Treaties 251 Duck and Cover 255 Let’s Nuke Des Moines! 265 Conclusion 270 Lest We Forget 274 The Beginning or The End? 280 Update: 7/1/12 Copyright © 2012 rbf 4 Preface 5 Hey there, I’m Ralph. That’s my dog Spot over there. Welcome to the not-so-wonderful world of nuclear weaponry. This book is a journey from 1945 when the first atomic bomb was detonated in the New Mexico desert to where we are today. It’s an interesting and sometimes bizarre journey. It can also be horribly frightening. Today, there are enough nuclear weapons to destroy the civilized world several times over. Over 23,000. “Enough to make the rubble bounce,” Winston Churchill said. The United States alone has over 10,000 warheads in what’s called the ‘enduring stockpile.’ In my time, we took care of things Mano-a-Mano. -
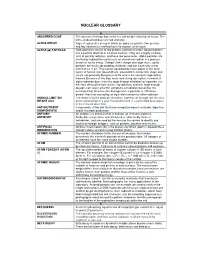
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Dosimetry of Low-Energy Beta Radiation
Risø-R-907(EN) Dosimetiy of Low-Energy Beta Radiation Jette Borg Risø National Laboratory, Roskilde, Denmark August 1996 VOL 2 7 »22 Dosimetry of Low-Energy Beta Radiation Jette Borg Risø National Laboratory, Roskilde, Denmark August 1996 Abstract Useful techniques and procedures for determination of absorbed doses from exposure in a low-energy /? radiation field were studied and evaluated in this project. The four different techniques included were /? spectrometry, extrapolation chamber dosimetry, Monte Carlo (MC) calculations, and exoelectron dosimetry. As a typical low-energy /? radiation field a moderated spectrum from a 14C source {Ep^max — 156 keV) was chosen for the study. The measured response of a Si(Li) detector to photons (bremsstrahlung) showed fine agreement with the MC calculated photon response, whereas the difference be- tween measured and MC calculated responses to electrons indicates an additional deadlayer thickness of about 12 /xm in the Si(Li) detector. The depth-dose profiles measured with extrapolation chambers at two labora- tories agreed very well, and it was confirmed that the fitting procedure previous- ly reported for 147Pm depth-dose profiles is also suitable for /? radiation from 14C. An increasing difference between measured and MC calculated dose rates for increasing absorber thicknesses was found, which is explained by limitations of the EGS4 code for transport of very low energy electrons (below 10 - 20 keV). Finally, a study of the thermally stimulated exoelectron emission (TSEE) re- sponse of BeO thin film dosemeters to /? radiation for radiation fields with maxi- mum P energies ranging from 67 keV to 2.27 MeV is reported. -

Rutherford Scattering
Rutherford Scattering MIT Department of Physics (Dated: September 24, 2014) This is an experiment which studies scattering alpha particles on atomic nuclei. You will shoot alpha particles, emitted by 241Am, at thin metal foils and measure the scattering cross section of the target atoms as a function of the scattering angle, the alpha particle energy, and the nuclear charge. You will then measure the intensity of alpha particles scattered by thin metal foils as a function of the scattering angle for several elements of very different atomic number. PREPARATORY QUESTIONS within which the electrons occupy certain positions of equilibrium, like raisins in a pudding. Set in motion, Please visit the Rutherford Scattering chapter on the the electrons should vibrate harmonically, radiating elec- 8.13x website at mitx.mit.edu to review the background tromagnetic energy with characteristic sharp frequencies material for this experiment. Answer all questions found that would be in the optical range if the radii of the −8 in the chapter. Work out the solutions in your laboratory atomic spheres were of the order of 10 cm. However, notebook; submit your answers on the web site. the \raisin pudding" model yielded no explanation of the numerical regularities of optical spectra, e.g. the Balmer formula for the hydrogen spectrum and the Ritz combi- SUGGESTED PROGRESS CHECK FOR END OF nation principle [3] for spectra in general. 2nd SESSION At this point, Ernest Rutherford got the idea that the structure of atoms could be probed by observing Plot the rate of alpha particle observations for 10◦ and the scattering of alpha particles. -

Development of a 2K Joule-Thomson Closed-Cycle Cryocooler
P# 1 81 Development of a 2K Joule-Thomson Closed-Cycle Cryocooler M. Crook, T. Bradshaw, G. Gilley, M. Hills, S. Watson, B. Green, C. Pulker, T. Rawlings STFC Rutherford Appleton Laboratory, Harwell, Oxford OX11 0QX ABSTRACT The Rutherford Appleton Laboratory (RAL) is currently developing a 2K Joule-Thomson cooler targeted at future space science missions requiring low temperatures, for example as part of the cryogenic chain for the ESA Athena X-ray telescope. The cooler builds on previous closed cycle cooler developments at RAL, in particular the very successful 4K-JT cooler that was flown on the ESA Planck mission. However the working fluid for the 2K-JT cooler will be 3He, and two extra stages of compression have been added to provide a much lower effluent pressure and a greater overall compression ratio. The design of the 2K-JT cooler is described, with reference to lessons learnt from Planck in-orbit data, and results of the test program to date are presented. INTRODUCTION Cryocooler development for space applications has been ongoing for over 30 years at RAL1, and has resulted in a range of closed cycle mechanical cryocoolers covering temperatures from 2 K to 80 K and above. These coolers have been licensed to both UK and US industry and have subsequently under- pinned a large variety of high profile scientific and operational missions; ranging from space exploration and the origins of the universe, to earth observation, climate change, and weather forecasting. One of those developments was a 4K Joule-Thomson (4K-JT) cooler2 which was flown on the ESA Planck mission3 to observe the fluctuations of the Cosmic Microwave Background. -

Effects of Nuclear Weapons Alexander Glaser Wws556d Princeton University February 12, 2007
Effects of Nuclear Weapons Alexander Glaser WWS556d Princeton University February 12, 2007 S. Glasstone and P. J. Dolan The Effects of Nuclear Weapons, Third Edition U.S. Government Printing Office Washington, D.C., 1977 1 Nuclear Weapon Tests USA Russia U.K. France China Total Atmo- 1945-63 1949-62 1952-58 1960-74 1964-80 528 spheric 215 219 21 50 23 Under- 1951-92 1961-90 1962-91 1961-96 1969-96 1517 ground 815 496 24 160 22 Total 1030 715 45 210 45 2045 India (1974, 1998): 1 + 5 Pakistan (1998): ca. 6 North Korea (2006): 1 2 3 Introduction / Overview 4 Burst Types • Air burst • High-altitude burst (above 100,000 ft) • Underwater burst • Underground burst • Surface burst In the following: primary focus on (medium-altitude) air bursts (fireball above surface, weak coupling into ground) 5 Effects of a Nuclear Explosion Typical distribution of energy released • Thermal radiation (including light) (35%) • Blast (pressure shock wave) (50%) • Nuclear radiation (prompt and delayed) (15%) 6 Effects of a Nuclear Explosion Sequence of events, Part I FIREBALL starts to form in less than a millionth of a second after explosion several tens of million of degrees: transformation of all matter into gas/plasma thermal radiation as x-rays, absorbed by the surrounding atmosphere for 1 Mt explosion : 440 ft in one millisecond, 5,700 ft in 10 seconds after one minute: cooled, no longer visible radiation Formation of the fireball triggers the destructive effects of the nuclear explosion 7 Trinity Test July 16, 1945 Shock front Fireball Mach front Dirt cloud -

Policymaking, Environmental Science, and the Nuclear Complex, 1945-1960
Splitting Atoms, Fracturing Landscapes: Policymaking, Environmental Science, and the Nuclear Complex, 1945-1960 BY ©2013 Neil Shafer Oatsvall Submitted to the graduate degree program in History and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. _________________________ Chairperson Sheyda Jahanbani _________________________ Gregory Cushman _________________________ Johannes Feddema _________________________ Sara Gregg _________________________ Theodore Wilson Date Defended: 7 December 2012 The Dissertation Committee for Neil Shafer Oatsvall certifies that this is the approved version of the following dissertation: Splitting Atoms, Fracturing Landscapes: Policymaking, Environmental Science, and the Nuclear Complex, 1945-1960 ________________________________ Chairperson Sheyda Jahanbani Date approved: 2 April 2013 ii ABSTRACT Neil S. Oatsvall, Ph.D. Department of History, May 2013 University of Kansas “Splitting Atoms, Fracturing Landscapes: Policymaking, Environmental Science, and the Nuclear Complex, 1945-1960” examines the implications of an expansive nuclear culture in the postwar United States. This dissertation probes the intersection of Cold War policymaking, environmental science, and the nuclear complex—a shorthand way of discussing the sum set of all nuclear technologies in conjunction with the societal structures and ideologies necessary to implement such technology. Studying a unified nuclear complex corrects for the limitations associated with studying all nuclear technologies as separate entities, something that has created fractured understandings of how splitting the atom affected both natural and human systems. This dissertation shows how U.S. policymakers in the early Cold War interacted with the environment and sought to fulfill their charge to protect the United States and its people while still attempting to ensure future national prosperity.