Rutherford Scattering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harry Moseley: Numbering the Elements
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 5. Harry Moseley: Numbering the Elements Dmitri Mendeleev (identified on screen) works on the Periodic Table, writes down the atomic weights of the elements. NARR: Previously on The Mystery of Matter… HISTORIAN MICHAEL GORDIN VO He figures out something rather extraordinary about the elements. DMITRI MENDELEEV, partly in VO The eye is immediately struck by a pattern within the horizontal rows and the vertical columns. Mendeleev’s first table morphs into the familiar modern Periodic Table. AUTHOR ERIC SCERRI VO He found an absolutely fundamental principle of nature. Humphry Davy (identified on screen) performs an experiment with his voltaic pile. HISTORIAN DAVID KNIGHT VO Somehow the particles of matter have to be glued together to form molecules. What Davy has had is a big idea. Perhaps electricity could be this kind of glue. Marie Curie (identified on screen) sits down at the spectroscope and peers into the eyepiece. NARR: The spectroscope kicked off a whole new round in the discovery of elements. The spectra of four elements appear on screen, along with their names. PHYSICIST DAVID KAISER VO It’s almost like each element has its own bar code. Marie and Pierre enter their lab at night and see vials of radium glowing on the shelves. MARIE TO PIERRE Don’t light the lamps! Look! 1 MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS PHYSICIST DAVID KAISER VO Radioactivity was a sign that the atom itself was unstable. It could break apart. Marie and Pierre look in wonder at their radiant element. NARR: Scientists now had a pressing new question to answer: What’s inside the atom? Fade to black ANNOUNCER: Major funding for The Mystery of Matter: Search for the Elements was provided by the National Science Foundation, where discoveries begin. -

Radiation and Radioactivity Quantified? Do You Think of These “People” When I Say RADIATION? Do You Think of These Things As Well?
Welcome To RadTown USA •Click to Explore RadTown USA • Click on any location in RadTown USA and find out about radiation sources or uses at that location. The Alpha, Beta, Gammas of Nuclear Education March 2nd, 2014 Fundamentals of Ionizing Radiation Debra N Thrall, PhD Executive Director Albert I Pierce Foundation Radiation Fundamentals What is radiation? Where does it come from? How does it interact with matter? What is radioactivity? What are fission and fusion? How are radiation and radioactivity quantified? Do you think of these “people” when I say RADIATION? Do you think of these things as well? • Food • Space • Utilities • Consumer Products • Medicine Brief History of the Atom • 500 BC Democritus Atom • Long time (Romans Dark Ages) • 1808 AD Dalton Plum Pudding • 1911 Rutherford Nucleus • 1913 Bohr Orbits • 1920’s Many People Quantum Mechanics Rutherford’s Gold Foil Experiment The Design 1. Bombard positively charged alpha particles into thin gold foil. 2. Use fluorescent screen to detect particles as they exit the gold foil. 3. Use angle of deflection to determine interior of the atom. So, What is an Atom? • Atoms are made up of protons, neutrons & electrons • Protons: + charge p+ • Neutrons: no charge n0 • Electrons: - charge e- • Atoms want to have a stable energy level • This translates to having no net charge • # protons = # electrons Mass of an Atom • Masses • Proton: 1.000000 amu • Neutron: 1.000000 amu • Electron: 0.000549 amu (Translates to 1.2 lbs/1 ton ~ a kitten on an elephant!) • The mass of an atom is approximately -

Rutherford's Atomic Model
CHAPTER 4 Structure of the Atom 4.1 The Atomic Models of Thomson and Rutherford 4.2 Rutherford Scattering 4.3 The Classic Atomic Model 4.4 The Bohr Model of the Hydrogen Atom 4.5 Successes and Failures of the Bohr Model 4.6 Characteristic X-Ray Spectra and Atomic Number 4.7 Atomic Excitation by Electrons 4.1 The Atomic Models of Thomson and Rutherford Pieces of evidence that scientists had in 1900 to indicate that the atom was not a fundamental unit: 1) There seemed to be too many kinds of atoms, each belonging to a distinct chemical element. 2) Atoms and electromagnetic phenomena were intimately related. 3) The problem of valence (원자가). Certain elements combine with some elements but not with others, a characteristic that hinted at an internal atomic structure. 4) The discoveries of radioactivity, of x rays, and of the electron Thomson’s Atomic Model Thomson’s “plum-pudding” model of the atom had the positive charges spread uniformly throughout a sphere the size of the atom with, the newly discovered “negative” electrons embedded in the uniform background. In Thomson’s view, when the atom was heated, the electrons could vibrate about their equilibrium positions, thus producing electromagnetic radiation. Experiments of Geiger and Marsden Under the supervision of Rutherford, Geiger and Marsden conceived a new technique for investigating the structure of matter by scattering particles (He nuclei, q = +2e) from atoms. Plum-pudding model would predict only small deflections. (Ex. 4-1) Geiger showed that many particles were scattered from thin gold-leaf targets at backward angles greater than 90°. -

Application of Ion Scattering Techniques to Characterize Polymer Surfaces and Interfaces
Materials Science and Engineering R 38 (2002) 107±180 Application of ion scattering techniques to characterize polymer surfaces and interfaces Russell J. Compostoa,*, Russel M. Waltersb, Jan Genzerc aLaboratory for Research on the Structure of Matter, Department of Materials Science and Engineering, University of Pennsylvania, Philadelphia, PA 19104-6272, USA bDepartment of Chemical Engineering, University of Pennsylvania, Philadelphia, PA 19104-6272, USA cDepartment of Chemical Engineering, North Carolina State University, Raleigh, NC 27695-7905, USA Abstract Ion beam analysis techniques, particularly elastic recoil detection (ERD) also known as forward recoil spectrometry (Frcs) has proven to be a value tool to investigate polymer surfaces and interfaces. A review of ERD, related techniques and their impact on the field of polymer science is presented. The physics of the technique is described as well as the underlying principles of the interaction of ions with matter. Methods for optimization of ERD for polymer systems are also introduced, specifically techniques to improve the depth resolution and sensitivity. Details of the experimental setup and requirements are also laid out. After a discussion of ERD, strategies for the subsequent data analysis are described. The review ends with the breakthroughs in polymer science that ERD enabled in polymer diffusion, surfaces, interfaces, critical phenomena, and polymer modification. # 2002 Elsevier Science B.V. All rights reserved. Keywords: Ion scattering; Polymer surface; Polymer interface; Elastic recoil detection; Forward recoil spectrometry; Depth profiling 1. Introduction Breakthroughs in polymer science typically correlate with the discovery and application of new experimental tools. One of the first examples was the prediction by P.J. Flory that the single chain conformation in a dense system (i.e. -
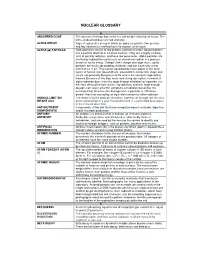
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Rutherford Scattering
Chapter 5 Lecture 4 Rutherford Scattering Akhlaq Hussain 1 5.5 Rutherford Scattering Formula Rutherford’s experiment of 훼-particles scattering by the atoms in a thin foil of gold revealed the existence of positively charged nucleus in the atom. ➢ The 훼-particles were scattered in all directions. ➢ Some passes undeflected by the foil. ➢ Some were scattered through larger angles even back scattered. ➢ The only valid explanation of large angle deflection was if the total positive charge were concentrated at some small region. ➢ Rutherford called it nucleus. 2 5.5 Rutherford Scattering Formula ′ 풖ퟏ 3 5.5 Rutherford Scattering Formula Consider an incident charge particle with charge z1e is scattered through a target with charge z2e. The trajectory of scattered particles is a hyperbola as shown. The trajectory of the particles in repulsive field with origin at o' is shown. In the case of attractive force, center will be at “o”. when particles are apart at large distance, the total energy it posses is in form of Kinetic energy ′ 1 ′ 2 푘 = ൗ2 µ푢1 = 퐸 ′ 2퐸 푢1 = µ 4 5.5 Rutherford Scattering Formula 푆 푑푠 We have to find 휎 휃′ = 푠푖푛 휃′ 푑휃′ ′ 푙 = µ푢1푆 = 푆 2µ퐸 Where S is the impact parameter. The trajectory is hyperbola, from equation of conic 훼 = 푒 cos 훼 − 1 and for 푟 → ∞ 푟 1 1 ⇒ cos 훼 = = 푒 2퐸푙2 1+ µ푘2 From the figure 2훼 + 휃′ = 휋 휋−휃′ 훼 = 2 휋−휃′ 휃′ Now cot 훼 = cot = tan 5 2 2 5.5 Rutherford Scattering Formula 휃′ cot 훼 = tan 2 휃′ cos 훼 cos 훼 tan = = 2 sin 훼 1−푐표푠2훼 휃′ cos 훼 tan = 2 1 cos 훼 −1 푐표푠2훼 휃′ 1 tan = 2 1 −1 푐표푠2훼 휃′ 1 1 tan = = 2 푒2−1 2퐸푙2 1+ −1 휇푘2 6 5.5 Rutherford Scattering Formula −1Τ 휃′ 2퐸푙2 2 tan = 2 휇푘2 휃′ 푘 휇 푘 tan = = ′ 2퐸 2 푙 2퐸 푙푢′ Because 푢 = 1 1 µ ′ Since 푙 = µ푢1S 휃′ 푘 ⇒ tan = ′2 2 푆µ푢1 Differentiating with θ' above equation can be written as. -

Dosimetry of Low-Energy Beta Radiation
Risø-R-907(EN) Dosimetiy of Low-Energy Beta Radiation Jette Borg Risø National Laboratory, Roskilde, Denmark August 1996 VOL 2 7 »22 Dosimetry of Low-Energy Beta Radiation Jette Borg Risø National Laboratory, Roskilde, Denmark August 1996 Abstract Useful techniques and procedures for determination of absorbed doses from exposure in a low-energy /? radiation field were studied and evaluated in this project. The four different techniques included were /? spectrometry, extrapolation chamber dosimetry, Monte Carlo (MC) calculations, and exoelectron dosimetry. As a typical low-energy /? radiation field a moderated spectrum from a 14C source {Ep^max — 156 keV) was chosen for the study. The measured response of a Si(Li) detector to photons (bremsstrahlung) showed fine agreement with the MC calculated photon response, whereas the difference be- tween measured and MC calculated responses to electrons indicates an additional deadlayer thickness of about 12 /xm in the Si(Li) detector. The depth-dose profiles measured with extrapolation chambers at two labora- tories agreed very well, and it was confirmed that the fitting procedure previous- ly reported for 147Pm depth-dose profiles is also suitable for /? radiation from 14C. An increasing difference between measured and MC calculated dose rates for increasing absorber thicknesses was found, which is explained by limitations of the EGS4 code for transport of very low energy electrons (below 10 - 20 keV). Finally, a study of the thermally stimulated exoelectron emission (TSEE) re- sponse of BeO thin film dosemeters to /? radiation for radiation fields with maxi- mum P energies ranging from 67 keV to 2.27 MeV is reported. -
Lecture 11 Ion Beam Analysis
1 AP 5301/8301 Instrumental Methods of Analysis and Laboratory Lecture 11 Ion Beam Analysis Prof YU Kin Man E-mail: [email protected] Tel: 3442-7813 Office: P6422 2 Lecture 8: outline . Introduction o Ion Beam Analysis: an overview . Rutherford backscattering spectrometry (RBS) o Introduction-history o Basic concepts of RBS – Kinematic factor (K) – Scattering cross-section – Depth scale o Quantitative thin film analysis o RBS data analysis . Other IBA techniques o Hydrogen Forward Scattering o Particle induced x-ray emission (PIXE) . Ion channeling o Minimum yield and critical angle o Dechanneling by defects o Impurity location 3 Analytical Techniques 100at% 10at% AES XRD IBA 1at% 0.1at% RBS XRF 100ppm TOF- 10ppm SIMS 1ppm TXRF 100ppb Detection Range Detection 10ppb Chemical bonding DynamicS 1ppb Elemental information IMS Imaging information 100ppt Thickness and density information 10ppt Analytical Spot size But IBA is typically quantitative and depth sensitive http://www.eaglabs.com 4 Ion Beam Analysis: an overview Inelastic Elastic Incident Ions Rutherford backscattering (RBS), resonant scattering, Nuclear Reaction Analysis channeling (NRA) p, a, n, g Particle induced x-ray emission (PIXE) Defects generation Elastic recoil detection Ion Beam Absorber foil Modification 5 Ion Beam Analysis 1977 2009 2014 1978 6 Why IBA? . Simple in principle . Fast and direct . Quantitative (without standard for RBS) . Depth profiling without chemical or physical sectioning . Non-destructive . Wide range of elemental coverage . No special specimen -

Rutherford Scattering
Acknowlegements: The E158, HAPPEX, PREX and MOLLER Collaborations www.particleadventure.org Electrons are Not Ambidextrous: New Insights from a Subatomic Matter of Fact Krishna Kumar Stony Brook University, SUNY Physics Colloquium BNL, November 4, 2014 Electroweak Nuclear Physics Outline ✦ Electron Scattering & Subatomic Structure ✦ Electron Scattering and the Weak Force ★ Electrons in the scattering process are NOT ambidextrous ✦ Parity-Violating Electron Scattering ✦ Two Modern Applications ★ The Neutron Skin of a Heavy Nucleus • PREX: First direct electroweak measurement (Phys.Rev.Lett. 108 (2012) 112502)! • PREX-II: Followup precision measurement scheduled for 2016! ★ Search for New Superweak Forces • MOLLER: Proposed ultra-precise weak mixing angle measurement! ✦ Outlook Electrons are Not Ambidextrous 2 Krishna Kumar, November 4 2014 Electroweak Nuclear Physics Outline Fundamental Symmetries, Nuclear Structure, Hadron Physics ✦ Electron Scattering & Subatomic Structure ✦ Electron Scattering and the Weak Force ★ Electrons in the scattering process are NOT ambidextrous ✦ Parity-Violating Electron Scattering ✦ Two Modern Applications ★ The Neutron Skin of a Heavy Nucleus • PREX: First direct electroweak measurement (Phys.Rev.Lett. 108 (2012) 112502)! • PREX-II: Followup precision measurement scheduled for 2016! ★ Search for New Superweak Forces • MOLLER: Proposed ultra-precise weak mixing angle measurement! ✦ Outlook Electrons are Not Ambidextrous 2 Krishna Kumar, November 4 2014 Electron Scattering and the Subatomic Structure ~ 1910 -

6. Basic Properties of Atoms
6. Basic properties of atoms ... We will only discuss several interesting topics in this chapter: Basic properties of atoms—basically, they are really small, really, really, really small • The Thomson Model—a really bad way to model the atom (with some nice E & M) • The Rutherford nuclear atom— he got the nucleus (mostly) right, at least • The Rutherford scattering distribution—Newton gets it right, most of the time • The Rutherford cross section—still in use today • Classical cross sections—yes, you can do this for a bowling ball • Spherical mirror cross section—the disco era revisited, but with science • Cross section for two unlike spheres—bowling meets baseball • Center of mass transformation in classical mechanics, a revised mass, effectively • Center of mass transformation in quantum mechanics—why is QM so strange? • The Bohr model—one wrong answer, one Nobel prize • Transitions in the Bohr model—it does work, I’m not Lyman to you • Deficiencies of the Bohr Model—how did this ever work in the first place? • After this we get back to 3D QM, and it gets real again. Elements of Nuclear Engineering and Radiological Sciences I NERS 311: Slide #1 ... Basic properties of atoms Atoms are small! e.g. 3 Iron (Fe): mass density, ρFe =7.874 g/cm molar mass, MFe = 55.845 g/mol N =6.0221413 1023, Avogadro’s number, the number of atoms/mole A × ∴ the volume occupied by an Fe atom is: MFe 1 45 [g/mol] 1 23 3 = 23 3 =1.178 10− cm NA ρ 6.0221413 10 [1/mol]7.874 [g/cm ] × Fe × The radius is (3V/4π)1/3 =0.1411 nm = 1.411 A˚ in Angstrøm units. -

Rutherford Scattering
IIA4 Modul Atomic/Nuclear Physics Rutherford Scattering This experiment aims at understanding the scattering experiments car- ried out by Ernest Rutherford and his colleagues. The observation of large-angle scattering of α-particles demonstrated how the electric charge is distributed inside the atoms. This experiment has significantly con- tributed to the understanding of the structure of matter. Experiment IIA4 - Rutherford Scattering This experiment aims at understanding the scattering experiments carried out by Ernest Ruther- ford and his colleagues. The observation of large-angle scattering of α-particles demonstrated how the electric charge is distributed inside the atoms. This experiment has significantly con- tributed to the understanding of the structure of matter. c AP, Department of Physics, University of Basel, June 2020 1.1 Preliminary Questions Describe the scattering experiment of Rutherford in your own words. • In this experiment, a so-called discriminator is used. What is it and why is it necessary? • Why is the experiment done in a vacuum chamber and not in air? • How does an oscilloscope function? What are its general settings? • 1.2 Theory 1.2.1 Development of the Atomic Model The first model of the atom was from the Greek philosophers Leucippus (around 440 BC) and his student Democritus (460-370 BC). They believed that all matter is made of small, indivisible particles. These particles were called atoms after the Greek word for indivisible. Between these atoms, in their view, only empty space would be found. Therefore, all macroscopic bodies and their similar or different properties would, by their arrangement, be determined. However, throughout the Middle Ages and up to the beginning of the modern era, this idea was again discarded because it was too materialistic and also disagreed with the doctrine of creation by the church. -

Development of a 2K Joule-Thomson Closed-Cycle Cryocooler
P# 1 81 Development of a 2K Joule-Thomson Closed-Cycle Cryocooler M. Crook, T. Bradshaw, G. Gilley, M. Hills, S. Watson, B. Green, C. Pulker, T. Rawlings STFC Rutherford Appleton Laboratory, Harwell, Oxford OX11 0QX ABSTRACT The Rutherford Appleton Laboratory (RAL) is currently developing a 2K Joule-Thomson cooler targeted at future space science missions requiring low temperatures, for example as part of the cryogenic chain for the ESA Athena X-ray telescope. The cooler builds on previous closed cycle cooler developments at RAL, in particular the very successful 4K-JT cooler that was flown on the ESA Planck mission. However the working fluid for the 2K-JT cooler will be 3He, and two extra stages of compression have been added to provide a much lower effluent pressure and a greater overall compression ratio. The design of the 2K-JT cooler is described, with reference to lessons learnt from Planck in-orbit data, and results of the test program to date are presented. INTRODUCTION Cryocooler development for space applications has been ongoing for over 30 years at RAL1, and has resulted in a range of closed cycle mechanical cryocoolers covering temperatures from 2 K to 80 K and above. These coolers have been licensed to both UK and US industry and have subsequently under- pinned a large variety of high profile scientific and operational missions; ranging from space exploration and the origins of the universe, to earth observation, climate change, and weather forecasting. One of those developments was a 4K Joule-Thomson (4K-JT) cooler2 which was flown on the ESA Planck mission3 to observe the fluctuations of the Cosmic Microwave Background.