Discovery of Radioactivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unerring in Her Scientific Enquiry and Not Afraid of Hard Work, Marie Curie Set a Shining Example for Generations of Scientists
Historical profile Elements of inspiration Unerring in her scientific enquiry and not afraid of hard work, Marie Curie set a shining example for generations of scientists. Bill Griffiths explores the life of a chemical heroine SCIENCE SOURCE / SCIENCE PHOTO LIBRARY LIBRARY PHOTO SCIENCE / SOURCE SCIENCE 42 | Chemistry World | January 2011 www.chemistryworld.org On 10 December 1911, Marie Curie only elements then known to or ammonia, having a water- In short was awarded the Nobel prize exhibit radioactivity. Her samples insoluble carbonate akin to BaCO3 in chemistry for ‘services to the were placed on a condenser plate It is 100 years since and a chloride slightly less soluble advancement of chemistry by the charged to 100 Volts and attached Marie Curie became the than BaCl2 which acted as a carrier discovery of the elements radium to one of Pierre’s electrometers, and first person ever to win for it. This they named radium, and polonium’. She was the first thereby she measured quantitatively two Nobel prizes publishing their results on Boxing female recipient of any Nobel prize their radioactivity. She found the Marie and her husband day 1898;2 French spectroscopist and the first person ever to be minerals pitchblende (UO2) and Pierre pioneered the Eugène-Anatole Demarçay found awarded two (she, Pierre Curie and chalcolite (Cu(UO2)2(PO4)2.12H2O) study of radiactivity a new atomic spectral line from Henri Becquerel had shared the to be more radioactive than pure and discovered two new the element, helping to confirm 1903 physics prize for their work on uranium, so reasoned that they must elements, radium and its status. -

Harry Moseley: Numbering the Elements
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 5. Harry Moseley: Numbering the Elements Dmitri Mendeleev (identified on screen) works on the Periodic Table, writes down the atomic weights of the elements. NARR: Previously on The Mystery of Matter… HISTORIAN MICHAEL GORDIN VO He figures out something rather extraordinary about the elements. DMITRI MENDELEEV, partly in VO The eye is immediately struck by a pattern within the horizontal rows and the vertical columns. Mendeleev’s first table morphs into the familiar modern Periodic Table. AUTHOR ERIC SCERRI VO He found an absolutely fundamental principle of nature. Humphry Davy (identified on screen) performs an experiment with his voltaic pile. HISTORIAN DAVID KNIGHT VO Somehow the particles of matter have to be glued together to form molecules. What Davy has had is a big idea. Perhaps electricity could be this kind of glue. Marie Curie (identified on screen) sits down at the spectroscope and peers into the eyepiece. NARR: The spectroscope kicked off a whole new round in the discovery of elements. The spectra of four elements appear on screen, along with their names. PHYSICIST DAVID KAISER VO It’s almost like each element has its own bar code. Marie and Pierre enter their lab at night and see vials of radium glowing on the shelves. MARIE TO PIERRE Don’t light the lamps! Look! 1 MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS PHYSICIST DAVID KAISER VO Radioactivity was a sign that the atom itself was unstable. It could break apart. Marie and Pierre look in wonder at their radiant element. NARR: Scientists now had a pressing new question to answer: What’s inside the atom? Fade to black ANNOUNCER: Major funding for The Mystery of Matter: Search for the Elements was provided by the National Science Foundation, where discoveries begin. -

The Radiochemistry of Tungsten
National Academy of Sciences !National Research Council NUCLEAR SCIENCE SERIES The Radiochemistry of Tungsten — ...—- L. F. C URTISS,Chairman ROBLEY D. EVANS, Vice Chairman NationalBureau ofStandards MassachusettsInstituteofTechnology J.A. DeJUREN, Secretary WestinghouseElectricCorporation C. J.BORKOWSKI J.W. IRVINE,JR. Oak RidgeNationalLaboratory MassachusettsI&tituteofTechnology ROBERT G. COCHRAN E. D. KLEMA Texas Agriculturaland Mechanical NorthwesternUniversity College W. WAYNE MEINKE SAMUEL EPSTEIN UniversityofMichigan CaliforniaInstituteofTechnology J.J.NICKSON Memorial Hospital,New York U. FANO NationalBureau ofStandards ROBERT L. PLATZMAN Laboratoirede Chimie Physique HERBERT GOLDSTEIN NuclearDevelopmentCorporationof D. M. VAN PATTER America BartolResearch Foundation LIAISON MEMBERS PAUL C. AEBERSOLD CHARLES K. REED Atomic Energy Commission U. S.Air Force J.HOWARD McMILLEN WILLIAM E. WRIGHT NationalScienceFoundation OfficeofNavalResearch SUBCOMMITTEE ON RADIOCHEMISTRY W. WAYNE MEINKE, Chai~man HAROLD KIRBY UniversityofMichigan Mound Laboratory GREGORY R. CHOPPIN GEORGE LEDDICOTTE FloridaStateUniversity Oak RidgeNationalLaboratory GEORGE A. COWAN JULIAN NIELSEN Los Alamos ScientificLaboratory HanfordLaboratories ARTHUR W. FAIRHALL ELLIS P. STEINBERG UniversityofWashington Argonne NationalLaboratory JEROME HUDIS PETER C. STEVENSON BrookhavenNationalLaboratory UniversityofCalifornia(Livermore) EARL HYDE LEO YAFFE UniversityofC slifornia(Berkeley) McGillUniversity CONSULTANTS NATHAN BALLOU JAMES DeVOE NavalRadiologicalDefenseLaboratory -

The Riches of Uranium Uranium Is Best Known, and Feared, for Its Involvement in Nuclear Energy
in your element The riches of uranium Uranium is best known, and feared, for its involvement in nuclear energy. Marisa J. Monreal and Paula L. Diaconescu take a look at how its unique combination of properties is now increasingly attracting the attention of chemists. t is nearly impossible to find an uplifting, and can be arrested by the skin, making found about uranium’s superior catalytic funny, or otherwise endearing quote on depleted uranium (composed mainly of 238U) activity may not be an isolated event. The Iuranium — the following dark wisecrack1 safe to work with as long as it is not inhaled organometallic chemistry of uranium was reflects people’s sinister feelings about this or ingested. born during the ‘Manhattan project’ — code element: “For years uranium cost only a few Studying the fundamental chemistry of name of the development of the first nuclear dollars a ton until scientists discovered you uranium is an exotic endeavour, but those who weapon during the Second World War. This could kill people with it”. But, in the spirit of embrace it will reap its benefits. Haber and field truly began to attract interest in 1956 rebranding, it is interesting to note that the Bosch found that uranium was a better catalyst when Reynolds and Wilkinson reported the main source of Earth’s internal heat comes than iron for making ammonia2. The preparation of the first cyclopentadienyl from the radioactive decay of uranium, isolation of an η1-OCO complex derivatives6. The discovery of thorium and potassium-40 that keeps the of uranium3 also showed uranocene electrified the field outer core liquid, induces mantle convection that, even though it is as much as that of ferrocene and, subsequently, drives plate tectonics. -

Radiation and Radioactivity Quantified? Do You Think of These “People” When I Say RADIATION? Do You Think of These Things As Well?
Welcome To RadTown USA •Click to Explore RadTown USA • Click on any location in RadTown USA and find out about radiation sources or uses at that location. The Alpha, Beta, Gammas of Nuclear Education March 2nd, 2014 Fundamentals of Ionizing Radiation Debra N Thrall, PhD Executive Director Albert I Pierce Foundation Radiation Fundamentals What is radiation? Where does it come from? How does it interact with matter? What is radioactivity? What are fission and fusion? How are radiation and radioactivity quantified? Do you think of these “people” when I say RADIATION? Do you think of these things as well? • Food • Space • Utilities • Consumer Products • Medicine Brief History of the Atom • 500 BC Democritus Atom • Long time (Romans Dark Ages) • 1808 AD Dalton Plum Pudding • 1911 Rutherford Nucleus • 1913 Bohr Orbits • 1920’s Many People Quantum Mechanics Rutherford’s Gold Foil Experiment The Design 1. Bombard positively charged alpha particles into thin gold foil. 2. Use fluorescent screen to detect particles as they exit the gold foil. 3. Use angle of deflection to determine interior of the atom. So, What is an Atom? • Atoms are made up of protons, neutrons & electrons • Protons: + charge p+ • Neutrons: no charge n0 • Electrons: - charge e- • Atoms want to have a stable energy level • This translates to having no net charge • # protons = # electrons Mass of an Atom • Masses • Proton: 1.000000 amu • Neutron: 1.000000 amu • Electron: 0.000549 amu (Translates to 1.2 lbs/1 ton ~ a kitten on an elephant!) • The mass of an atom is approximately -

Epistemology of Research on Radiation and Matter: a Structural View
Kairos. Journal of Philosophy & Science 22, 2019 Center for the Philosophy of Sciences of Lisbon University Epistemology of Research on Radiation and Matter: a Structural View Isabel Serra (CFCUL) ([email protected]) Elisa Maia (CFCUL e IIBRC) ([email protected]) DOI 10.2478/kjps-2019–0016 Abstract The modern understanding of radiation got its start in 1895 with X-rays discovered by Wilhelm Röntgen, followed in 1896 by Henri Becquerel’s discovery of radioactivity. The development of the study of radiation opened a vast field of resear- ch concerning various disciplines: chemistry, physics, biology, geology, sociology, ethics, etc. Additionally, new branches of knowledge were created, such as atomic and nuclear physics that enabled an in-depth knowledge of the matter. Moreover, during the historical evolution of this body of knowledge a wide variety of new te- chnologies was emerging. This article seeks to analyze the characteristics of expe- rimental research in radioactivity and microphysics, in particular the relationship experience-theory. It will also be emphasized that for more than two decades, since the discovery of radioactivity, experiments took place without the theory being able to follow experimental dynamics. Some aspects identified as structural features of scientific research in the area of radiation and matter will be addressed through his- torical examples. The inventiveness of experiments in parallel with the emergence of quantum mechanics, the formation of teams and their relationship with technology developed from the experiments, as well as the evolution of microphysics in the sen- se of “Big Science” will be the main structural characteristics here focused. The case study of research in radioactivity in Portugal that assumes a certain importance and has structural characteristics similar to those of Europe will be presented. -

Uranium Fact Sheet
Fact Sheet Adopted: December 2018 Health Physics Society Specialists in Radiation Safety 1 Uranium What is uranium? Uranium is a naturally occurring metallic element that has been present in the Earth’s crust since formation of the planet. Like many other minerals, uranium was deposited on land by volcanic action, dissolved by rainfall, and in some places, carried into underground formations. In some cases, geochemical conditions resulted in its concentration into “ore bodies.” Uranium is a common element in Earth’s crust (soil, rock) and in seawater and groundwater. Uranium has 92 protons in its nucleus. The isotope2 238U has 146 neutrons, for a total atomic weight of approximately 238, making it the highest atomic weight of any naturally occurring element. It is not the most dense of elements, but its density is almost twice that of lead. Uranium is radioactive and in nature has three primary isotopes with different numbers of neutrons. Natural uranium, 238U, constitutes over 99% of the total mass or weight, with 0.72% 235U, and a very small amount of 234U. An unstable nucleus that emits some form of radiation is defined as radioactive. The emitted radiation is called radioactivity, which in this case is ionizing radiation—meaning it can interact with other atoms to create charged atoms known as ions. Uranium emits alpha particles, which are ejected from the nucleus of the unstable uranium atom. When an atom emits radiation such as alpha or beta particles or photons such as x rays or gamma rays, the material is said to be undergoing radioactive decay (also called radioactive transformation). -

Download Report 2010-12
RESEARCH REPORt 2010—2012 MAX-PLANCK-INSTITUT FÜR WISSENSCHAFTSGESCHICHTE Max Planck Institute for the History of Science Cover: Aurora borealis paintings by William Crowder, National Geographic (1947). The International Geophysical Year (1957–8) transformed research on the aurora, one of nature’s most elusive and intensely beautiful phenomena. Aurorae became the center of interest for the big science of powerful rockets, complex satellites and large group efforts to understand the magnetic and charged particle environment of the earth. The auroral visoplot displayed here provided guidance for recording observations in a standardized form, translating the sublime aesthetics of pictorial depictions of aurorae into the mechanical aesthetics of numbers and symbols. Most of the portait photographs were taken by Skúli Sigurdsson RESEARCH REPORT 2010—2012 MAX-PLANCK-INSTITUT FÜR WISSENSCHAFTSGESCHICHTE Max Planck Institute for the History of Science Introduction The Max Planck Institute for the History of Science (MPIWG) is made up of three Departments, each administered by a Director, and several Independent Research Groups, each led for five years by an outstanding junior scholar. Since its foundation in 1994 the MPIWG has investigated fundamental questions of the history of knowl- edge from the Neolithic to the present. The focus has been on the history of the natu- ral sciences, but recent projects have also integrated the history of technology and the history of the human sciences into a more panoramic view of the history of knowl- edge. Of central interest is the emergence of basic categories of scientific thinking and practice as well as their transformation over time: examples include experiment, ob- servation, normalcy, space, evidence, biodiversity or force. -

Measuring Radioactivity
Health Physics Society Public Education Committee Fact Sheet MEASURING RADIOACTIVITY Because ionizing radiation cannot be detected with our human senses, we use various types of instruments and radiation detectors to measure the amount of radiation present. We usually measure both the amount of radioactivity in a radioisotope source, and the ionizing radiation field density being emitted by the source. We define radioactivity as the number of atoms which decay (disintegrate) in a radioisotope sample in a given period of time. The base unit is the Becquerel (Bq) or one disintegration per second (dps). This number is very small and therefore, not very useful. For this reason we use the Curie (Ci) which is 37 billion Bq. Because we often use very large or very small numbers when discussing radioactivity, we use a series o f prefixes which express multiples of 1000. The following table shows some of these prefixes: milli (m) = 1/1,000 kilo (k) = times 1,000 micro (u) = 1/1,000,000 mega (M) = times 1,000,000 nano (n) 1/1,000,000,000 giga (G) times 1,000,000,000 Pico (P) 1/1,000,000,000,000 tera (T) times 1,000,000,000,000 Using the table, a mCi = 1/1000 of a Curie and a GBq 1,000,000,000 Becquerels. To put this in perspective, a normal home smoke detector contains a small sealed source of about 10 uCi (370,000 Bq) of radioactivity. Ionizing radiation fields are expressed in units of Roentgens (R) which is equivalent to the number of atoms of a gas which are ionized. -

The International Commission on Radiological Protection: Historical Overview
Topical report The International Commission on Radiological Protection: Historical overview The ICRP is revising its basic recommendations by Dr H. Smith Within a few weeks of Roentgen's discovery of gamma rays; 1.5 roentgen per working week for radia- X-rays, the potential of the technique for diagnosing tion, affecting only superficial tissues; and 0.03 roentgen fractures became apparent, but acute adverse effects per working week for neutrons. (such as hair loss, erythema, and dermatitis) made hospital personnel aware of the need to avoid over- Recommendations in the 1950s exposure. Similar undesirable acute effects were By then, it was accepted that the roentgen was reported shortly after the discovery of radium and its inappropriate as a measure of exposure. In 1953, the medical applications. Notwithstanding these observa- ICRU recommended that limits of exposure should be tions, protection of staff exposed to X-rays and gamma based on consideration of the energy absorbed in tissues rays from radium was poorly co-ordinated. and introduced the rad (radiation absorbed dose) as a The British X-ray and Radium Protection Committee unit of absorbed dose (that is, energy imparted by radia- and the American Roentgen Ray Society proposed tion to a unit mass of tissue). In 1954, the ICRP general radiation protection recommendations in the introduced the rem (roentgen equivalent man) as a unit early 1920s. In 1925, at the First International Congress of absorbed dose weighted for the way different types of of Radiology, the need for quantifying exposure was radiation distribute energy in tissue (called the dose recognized. As a result, in 1928 the roentgen was equivalent in 1966). -

Laboratoire National Henri Becquerel Ccri(I)/01-05 Dimri/Lnhb/Bc/01-146 30/03/01
BUREAU NATIONAL DE MÉTROLOGIE COMMISSARIAT À L'ÉNERGIE ATOMIQUE LABORATOIRE NATIONAL HENRI BECQUEREL CCRI(I)/01-05 DIMRI/LNHB/BC/01-146 30/03/01 BUREAU NATIONAL DE METROLOGIE Laboratoire National Henri Becqurel (BNM-LNHB) Laboratoire Central des Industries Electriques (BNM-LCIE) DOSIMETRY OF PHOTONS AND CHARGED PARTICLES Progress Report 2000-2001 B.Chauvenet Participation in the CCRI K4 key comparison on calibration factors of ionisation chambers in terms of absorbed dose to water for 60Co photons Comparison of standards of absorbed dose to water for high-energy photon beams with METAS A comparison was carried out with METAS in October 2000. This comparison dealt with air kerma and absorbed dose to water standards in 60Co beams, and absorbed dose to water standards for high-energy x rays from accelerators (6 MV, 12 MV and 20 MV). The comparison was carried out in BNM-LNHB beams (60Co and Saturne 43 accelerator), with transfer chambers from METAS. Results are under analysis. Comparison of standards of absorbed dose to water for high-energy photon beams with NRC This comparison was carried out in October 1998. The results were discussed and analysed, and will be presented in a paper which has been submitted to “Physics in Medicine and Biology”. EUROMET projects Participation in EUROMET Contact Persons meetings for the preparation of CMC tables. The laboratory will participate in the proposed project of METAS on quality factors for high-energy photon beams. Absorbed dose to graphite by calorimetry The realisation of a new graphite calorimeter is under study to replace the present one built in 1984. -
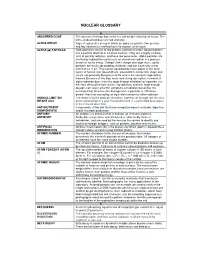
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit .