Experiment 3 Radioactivity: Effect of Distance and Absorbers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harry Moseley: Numbering the Elements
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 5. Harry Moseley: Numbering the Elements Dmitri Mendeleev (identified on screen) works on the Periodic Table, writes down the atomic weights of the elements. NARR: Previously on The Mystery of Matter… HISTORIAN MICHAEL GORDIN VO He figures out something rather extraordinary about the elements. DMITRI MENDELEEV, partly in VO The eye is immediately struck by a pattern within the horizontal rows and the vertical columns. Mendeleev’s first table morphs into the familiar modern Periodic Table. AUTHOR ERIC SCERRI VO He found an absolutely fundamental principle of nature. Humphry Davy (identified on screen) performs an experiment with his voltaic pile. HISTORIAN DAVID KNIGHT VO Somehow the particles of matter have to be glued together to form molecules. What Davy has had is a big idea. Perhaps electricity could be this kind of glue. Marie Curie (identified on screen) sits down at the spectroscope and peers into the eyepiece. NARR: The spectroscope kicked off a whole new round in the discovery of elements. The spectra of four elements appear on screen, along with their names. PHYSICIST DAVID KAISER VO It’s almost like each element has its own bar code. Marie and Pierre enter their lab at night and see vials of radium glowing on the shelves. MARIE TO PIERRE Don’t light the lamps! Look! 1 MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS PHYSICIST DAVID KAISER VO Radioactivity was a sign that the atom itself was unstable. It could break apart. Marie and Pierre look in wonder at their radiant element. NARR: Scientists now had a pressing new question to answer: What’s inside the atom? Fade to black ANNOUNCER: Major funding for The Mystery of Matter: Search for the Elements was provided by the National Science Foundation, where discoveries begin. -

Radiation and Radioactivity Quantified? Do You Think of These “People” When I Say RADIATION? Do You Think of These Things As Well?
Welcome To RadTown USA •Click to Explore RadTown USA • Click on any location in RadTown USA and find out about radiation sources or uses at that location. The Alpha, Beta, Gammas of Nuclear Education March 2nd, 2014 Fundamentals of Ionizing Radiation Debra N Thrall, PhD Executive Director Albert I Pierce Foundation Radiation Fundamentals What is radiation? Where does it come from? How does it interact with matter? What is radioactivity? What are fission and fusion? How are radiation and radioactivity quantified? Do you think of these “people” when I say RADIATION? Do you think of these things as well? • Food • Space • Utilities • Consumer Products • Medicine Brief History of the Atom • 500 BC Democritus Atom • Long time (Romans Dark Ages) • 1808 AD Dalton Plum Pudding • 1911 Rutherford Nucleus • 1913 Bohr Orbits • 1920’s Many People Quantum Mechanics Rutherford’s Gold Foil Experiment The Design 1. Bombard positively charged alpha particles into thin gold foil. 2. Use fluorescent screen to detect particles as they exit the gold foil. 3. Use angle of deflection to determine interior of the atom. So, What is an Atom? • Atoms are made up of protons, neutrons & electrons • Protons: + charge p+ • Neutrons: no charge n0 • Electrons: - charge e- • Atoms want to have a stable energy level • This translates to having no net charge • # protons = # electrons Mass of an Atom • Masses • Proton: 1.000000 amu • Neutron: 1.000000 amu • Electron: 0.000549 amu (Translates to 1.2 lbs/1 ton ~ a kitten on an elephant!) • The mass of an atom is approximately -

AFRRI Biodosimetry Worksheet
Armed Forces Radiobiology Research Institute Biodosimetry Worksheet (Medical Record of Radiation Dose, Contamination, and Acute Radiation Sickness Response) Reporting Authority (person(s) creating this page of the report) Last name: First name: Country of origin: Unit: Phone: Fax: Email: Location: Date (yymmdd): Time: Casualty Last name: First name: Rank: Country of origin: Parent unit: Parent unit location: Parent unit phone: Unit e-mail: Unit fax: Casualty location: History of presenting injury (conventional and/or radiation): History of previous radiation exposure: Past medical history (general): Medical countermeasures (e.g., antiemetics, transfusion), specify: Administered (where, when, route): Exposure conditions Date of exposure (yymmdd): Exposure location: Time of exposure: Weather conditions (at time of exposure): Exposure results Describe incident: External exposure overview Contamination overview Body exposure: Total Partial Uncertain External contamination: Yes No Shielding confounder: Yes No Internal contamination: Yes No Contaminated wound: Yes No If wound(s) are radiation contaminated, please provide details here: Biodosimetric assays overview Sampling date, time Estimated time Dose Reference radiation quality yymmdd (time) post-exposure (h) (Gy) and dose rate (Gy/min) Time onset of vomiting: Lymphocyte counts or depletion kinetics: Urine bioassay: Cytogenetic biodosimetry: Other: ARS response category overview (maximum grading 0-4; see pages 4 through 6 for guidance) N: C: G: H: = RC: days after radiation exposure: AFRRI -

Digital Radiation Monitor Is a Health and Safety Instrument That Measures Alpha, Digital Radiation Monitor Beta, and Gamma Radiation
1 Introduction The Digital Radiation Monitor is a health and safety instrument that measures alpha, Digital Radiation Monitor beta, and gamma radiation. With the Digital Radiation Monitor, you can: • Monitor possible radiation exposure while working near radionuclides (Order Code DRM-BTD) • Ensure compliance with regulatory standards Contents • Check for leakage from X-ray machines and other sources • Screen for environmental contamination or environmental sources of 1 Introduction ............................................................................................................. 2 radioactivity How the DRM Detects Radiation ................................................................. 2 • Connect the Digital Radiation Monitor to a computer or data logger to record 2 Features ................................................................................................................... 3 and tabulate your data The Display................................................................................................... 3 The Switches ................................................................................................4 This manual gives complete instructions for using the Digital Radiation Monitor and The Detector..................................................................................................5 procedures for common applications. The Ports....................................................................................................... 5 3 Operation................................................................................................................ -
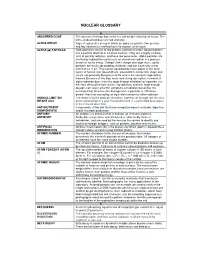
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Dosimetry of Low-Energy Beta Radiation
Risø-R-907(EN) Dosimetiy of Low-Energy Beta Radiation Jette Borg Risø National Laboratory, Roskilde, Denmark August 1996 VOL 2 7 »22 Dosimetry of Low-Energy Beta Radiation Jette Borg Risø National Laboratory, Roskilde, Denmark August 1996 Abstract Useful techniques and procedures for determination of absorbed doses from exposure in a low-energy /? radiation field were studied and evaluated in this project. The four different techniques included were /? spectrometry, extrapolation chamber dosimetry, Monte Carlo (MC) calculations, and exoelectron dosimetry. As a typical low-energy /? radiation field a moderated spectrum from a 14C source {Ep^max — 156 keV) was chosen for the study. The measured response of a Si(Li) detector to photons (bremsstrahlung) showed fine agreement with the MC calculated photon response, whereas the difference be- tween measured and MC calculated responses to electrons indicates an additional deadlayer thickness of about 12 /xm in the Si(Li) detector. The depth-dose profiles measured with extrapolation chambers at two labora- tories agreed very well, and it was confirmed that the fitting procedure previous- ly reported for 147Pm depth-dose profiles is also suitable for /? radiation from 14C. An increasing difference between measured and MC calculated dose rates for increasing absorber thicknesses was found, which is explained by limitations of the EGS4 code for transport of very low energy electrons (below 10 - 20 keV). Finally, a study of the thermally stimulated exoelectron emission (TSEE) re- sponse of BeO thin film dosemeters to /? radiation for radiation fields with maxi- mum P energies ranging from 67 keV to 2.27 MeV is reported. -

Rutherford Scattering
Rutherford Scattering MIT Department of Physics (Dated: September 24, 2014) This is an experiment which studies scattering alpha particles on atomic nuclei. You will shoot alpha particles, emitted by 241Am, at thin metal foils and measure the scattering cross section of the target atoms as a function of the scattering angle, the alpha particle energy, and the nuclear charge. You will then measure the intensity of alpha particles scattered by thin metal foils as a function of the scattering angle for several elements of very different atomic number. PREPARATORY QUESTIONS within which the electrons occupy certain positions of equilibrium, like raisins in a pudding. Set in motion, Please visit the Rutherford Scattering chapter on the the electrons should vibrate harmonically, radiating elec- 8.13x website at mitx.mit.edu to review the background tromagnetic energy with characteristic sharp frequencies material for this experiment. Answer all questions found that would be in the optical range if the radii of the −8 in the chapter. Work out the solutions in your laboratory atomic spheres were of the order of 10 cm. However, notebook; submit your answers on the web site. the \raisin pudding" model yielded no explanation of the numerical regularities of optical spectra, e.g. the Balmer formula for the hydrogen spectrum and the Ritz combi- SUGGESTED PROGRESS CHECK FOR END OF nation principle [3] for spectra in general. 2nd SESSION At this point, Ernest Rutherford got the idea that the structure of atoms could be probed by observing Plot the rate of alpha particle observations for 10◦ and the scattering of alpha particles. -

Radiation Glossary
Radiation Glossary Activity The rate of disintegration (transformation) or decay of radioactive material. The units of activity are Curie (Ci) and the Becquerel (Bq). Agreement State Any state with which the U.S. Nuclear Regulatory Commission has entered into an effective agreement under subsection 274b. of the Atomic Energy Act of 1954, as amended. Under the agreement, the state regulates the use of by-product, source, and small quantities of special nuclear material within said state. Airborne Radioactive Material Radioactive material dispersed in the air in the form of dusts, fumes, particulates, mists, vapors, or gases. ALARA Acronym for "As Low As Reasonably Achievable". Making every reasonable effort to maintain exposures to ionizing radiation as far below the dose limits as practical, consistent with the purpose for which the licensed activity is undertaken. It takes into account the state of technology, the economics of improvements in relation to state of technology, the economics of improvements in relation to benefits to the public health and safety, societal and socioeconomic considerations, and in relation to utilization of radioactive materials and licensed materials in the public interest. Alpha Particle A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. It is identical to a helium nucleus, with a mass number of 4 and a charge of +2. Annual Limit on Intake (ALI) Annual intake of a given radionuclide by "Reference Man" which would result in either a committed effective dose equivalent of 5 rems or a committed dose equivalent of 50 rems to an organ or tissue. Attenuation The process by which radiation is reduced in intensity when passing through some material. -

Ranger Operating Manual
INTERNATIONAL ® S.E. International, Inc. P.O. Box 39, 436 Farm Rd. Summertown, TN 38483 USA 1.800.293.5759 | 931.964.3561 | Fax: 1.931.964.3564 www.seintl.com | [email protected] Contents Chapter 1: Introduction 3 Preferences 14 How The unit Detects Radiation 3 Using the Data Logging Feature 14 Precautions 3 Show Grid 15 Observer USB Chart Screen 15 Chapter 2: Features 4 The X Axis 15 The LCD Display 4 The Y Axis 15 Indicators 4 Observer USB Meter Screen 15 The Buttons 5 Enable Alarm 15 Power Button 5 Zero 15 Alarm Button 5 Units/Echo Display 15 Count Button 5 Cal Panel (Calibration Panel) 16 Audio Button 5 Calibration Information 16 Menu Button 5 Applied Isotope 16 Backlight Button 5 Alarm Settings 16 Mode Button 6 Preset Counting Time (min) 16 The Detector 6 Data Logging Settings 16 USB Jack 6 Backlight On Time 16 Lanyard 6 Auto-Averaging 16 Xtreme Boot 6 Audio Settings 16 Chapter 3: Operation 7 Starting The unit 7 Chapter 8: Built in Isotope Efficiencies 17 Units of Measurement 7 Built in Isotope Efficiencies 17 Display Update 7 Decay 17 Maximum level 7 Selecting a Built-In Isotope Efficiency 17 Response Time (Autoaveraging) 7 Adding A Custom Isotope Efficiency 17 Autoranging 8 Operating in Dose/Rate Modes 8 Chapter 9: Observer USB Calibration Software 18 Using The Alarm 8 General Discussion of Calibration 18 Operating in Count Mode 9 Pulse Based Pre-Calibration 18 How To Take A Timed Count 9 Efficiency Calibration 19 Using Dose/Rate Modes While Timer is On 9 Exposure Rate Calibration 20 The Menu 10 Menu Options 10 Chapter 10: Troubleshooting 22 Setting the Internal Clock 10 Interfacing with an External Device 10 Chapter 11: Basics of Taking Measurements 23 How to Detect Background Radiation 23 Chapter 4: Common Procedures 11 How To Survey a Surface 23 Establishing the Background Count 11 How to Perform a General Survey 23 Environmental Area Monitoring 11 How to Determine Alpha, Beta, or Gamma source. -

Development of a 2K Joule-Thomson Closed-Cycle Cryocooler
P# 1 81 Development of a 2K Joule-Thomson Closed-Cycle Cryocooler M. Crook, T. Bradshaw, G. Gilley, M. Hills, S. Watson, B. Green, C. Pulker, T. Rawlings STFC Rutherford Appleton Laboratory, Harwell, Oxford OX11 0QX ABSTRACT The Rutherford Appleton Laboratory (RAL) is currently developing a 2K Joule-Thomson cooler targeted at future space science missions requiring low temperatures, for example as part of the cryogenic chain for the ESA Athena X-ray telescope. The cooler builds on previous closed cycle cooler developments at RAL, in particular the very successful 4K-JT cooler that was flown on the ESA Planck mission. However the working fluid for the 2K-JT cooler will be 3He, and two extra stages of compression have been added to provide a much lower effluent pressure and a greater overall compression ratio. The design of the 2K-JT cooler is described, with reference to lessons learnt from Planck in-orbit data, and results of the test program to date are presented. INTRODUCTION Cryocooler development for space applications has been ongoing for over 30 years at RAL1, and has resulted in a range of closed cycle mechanical cryocoolers covering temperatures from 2 K to 80 K and above. These coolers have been licensed to both UK and US industry and have subsequently under- pinned a large variety of high profile scientific and operational missions; ranging from space exploration and the origins of the universe, to earth observation, climate change, and weather forecasting. One of those developments was a 4K Joule-Thomson (4K-JT) cooler2 which was flown on the ESA Planck mission3 to observe the fluctuations of the Cosmic Microwave Background. -

PHYS 5012 Radiation Physics and Dosimetry
Radiation Physics Lecture 1 Background and Fundamentals The Discovery of Radiation X-rays Radioactivity Classification of Radiation Types of Ionising Radiation PHYS 5012 Radiation Units and Properties Radiation Physics and Dosimetry Dose in Water Atomic Physics and Radiation Lecture 1 The Rutherford-Bohr Model Multi-Electron Atoms Production of Radiation Characteristic Radiation Characteristic X-rays Auger Electrons Continuous Radiation Bremsstrahlung Radiation Tuesday 5 March 2013 Synchrotron Radiation Cerenkov Radiation Particle Accelerators X-ray Tubes Cyclotrons Linear Accelerators Radiation Physics Lecture 1 The Discovery of Radiation Background and Fundamentals The Discovery of Radiation X-rays Radioactivity Classification of Radiation Types of Ionising Radiation Three main discoveries of radiation made at the turn of Radiation Units and Properties the 19th century, together with several major advances in Dose in Water Atomic Physics and theoretical physics, including quantum mechanics and Radiation The Rutherford-Bohr special relativity, signalled the birth of Radiation Physics. Model Multi-Electron Atoms The subsequent realisation that radiation can be harmful Production of Radiation to humans led to the the rapid development of radiation Characteristic Radiation Characteristic X-rays dosage measurements and quantification and commonly Auger Electrons accepted standards for tolerable levels of radiation in Continuous Radiation Bremsstrahlung Radiation humans. Synchrotron Radiation Cerenkov Radiation Particle Accelerators X-ray Tubes -

Quantum Mechanics and Particle Scattering
QuantumQuantum Mechanics Mechanics and and Particle Particle Scattering Scattering Lecture 1 • Introduction to the Course • Scales and Units • Rutherford Scattering - 1911 • The New Physics - Quantum Mechanics Eram Rizvi Royal Institution - London 7th February 2012 Who Am I ? Always wanted to be a physicist Heard about discovery of two new particles the W and Z in 1981 (BBC2 Horizon) Wanted to be a particle physicist ever since I now work with some people featured in that programme Studied Physics at Manchester University Graduated 1st class in 1993 PhD in Particle Physics from Queen Mary, London Joined new collider experiment: HERA - high energy and unique electron-proton accelerator in Hamburg Awarded PhD in 1997 Research Fellow at HERA laboratory - measured quark structure Postdoc with Birmingham University - precision proton structure measurements Lecturer at Queen Mary, London - teaching Nuclear Physics, Scientific Measurement, undergraduate tutorials Tutor for undergraduate admissions to Physics Postgraduate admissions tutor for Particle Physics research group Research focus in 3 areas: - leading team of researchers finalising measurements of proton structure from HERA (2 months to go!) - joined Atlas experiment on LHC - co-ordinating a measurement of quark/gluon dynamics - author and project leader of team producing state-of-the-art simulations for micro-black holes at LHC - starting involvement to design a ‘trigger’ system for an upgrade to the LHC in 2018 These are my dream jobs! Eram Rizvi Lecture 1 - Royal Institution - London