The Ecological Effects of Providing Resource Subsidies to Predators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fur Trade and the Biotic Homogenization of Subpolar Ecosystems
Chapter 14 Fur Trade and the Biotic Homogenization of Subpolar Ecosystems Ramiro D. Crego, Ricardo Rozzi, and Jaime E. Jiménez Abstract At the southern end of the Americas exist one of the last pristine ecosys- tems in the world, the sub-Antarctic Magellanic forests ecoregion, protected by the Cape Horn Biosphere Reserve (CHBR). Despite its remote location, the CHBR has been subject to the growing infuences of globalization, a process that has driven cultural, biotic, and economic transformations in the region since the mid-twentieth century. One of the most important threats to these unique ecosystems is the increase of biological invasions. Motivated by the expanding fur industry that responded to the globalization process, American beavers (Castor canadensis), muskrats (Ondatra zibethicus), and American minks (Neovison vison) were introduced, inde- pendently, to the southern tip of South America. Research has shown that these three North American species have reassembled their native interactions to affect negatively the invaded ecosystems of the CHBR. Beavers affect river fow and R. D. Crego (*) Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA R. Rozzi Department of Philosophy and Religion and Department of Biological Sciences, University of North Texas, Denton, TX, USA Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad and Universidad de Magallanes, Punta Arenas, Chile J. E. Jiménez Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Department of Philosophy and Religion, University of North Texas, Denton, TX, USA Universidad de Magallanes, Punta Arenas, Chile © Springer Nature Switzerland AG 2018 233 R. -
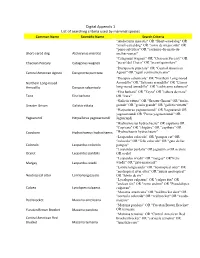
Digital Appendix 1 List of Searching Criteria Used by Mammal Species
Digital Appendix 1 List of searching criteria used by mammal species Common Name Scientific Name Search Criteria "Atelocynus microtis" OR "Short-eared dog" OR "small-eared dog" OR "zorro de oreja corta" OR "perro selvático" OR "cachorro-do-mato-de- Short-eared dog Atelocynus microtis orelhas-curtas" "Catagonus wagneri" OR "Chacoan Peccary" OR Chacoan Peccary Catagonus wagneri "pecarí del Chaco" OR "pecarí quimilero" “Dasyprocta punctate” OR "Central American Central American Agouti Dasyprocta punctata Agouti" OR "agutí centroamericano" “Dasypus sabanicola” OR "Northern Long-nosed Northern Long-nosed Armadillo" OR "Savanna armadillo" OR "Llanos Armadillo Dasypus sabanicola long-nosed armadillo" OR "cachicamo sabanero" “Eira barbara” OR "Tayra" OR "cabeza de mate" Taira Eira barbara OR "irara" “Galictis vittata” OR "Greater Grison" OR "furão- Greater Grison Galictis vittata grande" OR "grisón grande" OR "galictis vittatta" “Herpailurus yagouaroundi” OR Yaguarundi OR yagouaroundi OR "Puma yagouaroundi" OR Yaguarundi Herpailurus yagouaroundi Jaguarundi "Hydrochoerus hydrochaeris" OR capybara OR "Capivara" OR "chigüire" OR "capibara" OR Capybara Hydrochoerus hydrochaeris "Hydrochaeris hydrochaeris" “Leopardus colocolo” OR "pampas cat" OR "colocolo" OR "felis colocolo" OR "gato de las Colocolo Leopardus colocolo pampas" "Leopardus pardalis" OR jaguatirica OR ocelote Ocelot Leopardus pardalis OR ocelot “Leopardus wiedii” OR "margay" OR"Felis Margay Leopardus wiedii wiedii" OR "gato-maracajá" “Lontra longicaudis” OR "neotropical otter" OR "neotropical -

Temporal Changes in the Diet of Two Sympatric Carnivorous Mammals in a Protected Area of South–Central Chile Affected by a Mixed–Severity Forest Fire
Animal Biodiversity and Conservation 43.2 (2020) 177 Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire A. H. Zúñiga, J. R. Rau, V. Fuenzalida, A. Fuentes–Ramírez Zúñiga, A. H., Rau, J. R., Fuenzalida, V., Fuentes–Ramírez, A., 2020. Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire. Animal Biodiversity and Conservation, 43.2: 177–186, Doi: https://doi.org/10.32800/abc.2020.43.0177 Abstract Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire. Fire is a significant disruptive agent in various ecosystems around the world. It can affect the availability of resources in a given area, modulating the interaction between competing species. We studied the diet of the culpeo fox (Lycalopex culpaeus) and cougar (Puma concolor) for two consecutive years in a protected area of southern–central Chile which was affected by a wildfire. Significant differences were observed in the dietary pattern between the two species, showing their trophic segregation. In the two years of the study, the predominant prey for cougar was an exotic species, the European hare (Lepus europaeus), implying a simplification of its trophic spectrum with respect to that reported in other latitudes. The ecological consequences related to this scenario are discussed. Key words: Dietary overlap, Predation, Post–fire dynamics, Microhabitat, Rodent cycles, Selectivity Resumen Cambios temporales en la dieta de dos mamíferos carnívoros simpátridas en una zona protegida del centro y el sur de Chile afectada por un incendio forestal de intensidad desigual. -

Activity Patterns in Sympatric Carnivores in the Nahuelbuta Mountain Range, Southern-Central Chile
Mammalia 2016; aop Alfredo H. Zúñiga*, Jaime E. Jiménez and Pablo Ramírez de Arellano Activity patterns in sympatric carnivores in the Nahuelbuta Mountain Range, southern-central Chile DOI 10.1515/mammalia-2015-0090 Received June 24, 2015; accepted August 26, 2016 Introduction Abstract: Species interactions determine the structure of Species interactions are one of the most studied topics in biological communities. In particular, interference behav- community ecology, as interspecific behavior can largely ior is critical as dominant species can displace subordi- determine the composition and structure of community nate species depending on local ecological conditions. In assemblages (Case and Gilpin 1974). For carnivores, inter- carnivores, the outcome of interference may have impor- specific interactions are particularly relevant because of tant consequences from the point of view of conservation, their role in top-down control in terrestrial ecosystems especially when vulnerable species are the ones suffering (Terborgh and Winter 1980). Nevertheless, given the key displacement. Using 24 baited camera traps and a sam- role of consumers and through trophic cascades, changes pling effort of 2821 trap nights, we examined the activ- in the environment could promote an increase of medium- ity patterns and spatial overlap of an assemblage of five sized carnivores or mesopredators, due to top predator sympatric carnivores in the Nahuelbuta Mountain Range, removal (Prange and Gehrt 2007) which can cause sub- in southern-central Chile. In this forested landscape we stantial changes in the dynamics of interaction among found predominantly nocturnal activity in all species, but sympatric species (Kamler et al. 2013), with adverse not for the puma (Puma concolor) and to a lesser extent, effects on subordinate species. -

Report of the Presence of Wild Animals
Report of the Presence of Wild Animals The information recorded here is essential to emergency services personnel so that they may protect themselves and your neighbors, provide for the safety of your animals, ensure the maximum protection and preservation of your property, and provide you with emergency services without unnecessary delay. Every person in New York State, who owns, possesses, or harbors a wild animal, as set forth in General Municipal Law §209-cc, must file this Report annually, on or before April 1, of each year, with the clerk of the city, village or town (if outside a village) where the animal is kept. A list of the common names of animals to be reported is enclosed with this form. Failure to file as required will subject you to penalties under law. A separate Report is required to be filed annually for each address where a wild animal is harbored. Exemptions: Pet dealers, as defined in section 752-a of the General Business Law, zoological facilities and other exhibitors licensed pursuant to U.S. Code Title 7 Chapter 54 Sections 2132, 2133 and 2134, and licensed veterinarians in temporary possession of dangerous dogs, are not required to file this report. Instructions for completing this form: 1. Please print or type all information, using blue or black ink. 2. Fill in the information requested on this page. 3. On the continuation sheets, fill in the information requested for each type of animal that you possess. 4. Return the completed forms to the city, town, or village clerk of each municipality where the animal or animals are owned, possessed or harbored. -

How Are Wetlands and Biological Interactions Related to Carnivore Distributions at High Altitude?
Journal of Arid Environments 115 (2015) 14e18 Contents lists available at ScienceDirect Journal of Arid Environments journal homepage: www.elsevier.com/locate/jaridenv Short communication How are wetlands and biological interactions related to carnivore distributions at high altitude? * G.A.E. Cuyckens a, b, , P.G. Perovic c, L. Cristobal d a Consejo Nacional de Investigaciones Científicas y Tecnicas (CONICET), Argentina b Centro de Estudios Territoriales, Ambientales y Sociales (CETAS), Facultad de Ciencias Agrarias, Universidad Nacional de Jujuy, Argentina c Administracion de Parques Nacionales, Delegacion Noroeste, Argentina d Fundacion ProYungas, Argentina article info abstract Article history: Determining the geographic range of species is a main objective in ecology and has implications for Received 20 December 2013 conservation. Key determinants of carnivore distribution in dry environments are competition and the Received in revised form availability of water. Here, we gathered and mapped the available information on carnivore habitat 18 December 2014 quality in the high Andes and Puna in the extreme north of Argentina. We investigated four carnivore Accepted 23 December 2014 species: the Andean cat (Leopardus jacobita), the Pampas cat (Leopardus colocolo), the cougar (Puma Available online concolor) and the culpeo fox (Lycalopex culpaeus). We assessed the main determinants of their distri- bution, testing explicitly for the effects of seasonal and temporal wetlands and biological interactions. We Keywords: Competition used species distribution models, and created biophysical models using environmental and landscape High Andes variables. Then, by including the four species' biophysical models into the model of the focal species, we Leopardus jacobita tested for the importance of biological interactions. Wetlands were most important for the culpeo fox, L. -

Ebook Download Seals and Sea Lions Ebook
SEALS AND SEA LIONS PDF, EPUB, EBOOK John Crossingham,Bobbie Kalman | 32 pages | 28 Feb 2006 | Crabtree Publishing Co,Canada | 9780778713234 | English | New York, Canada Seals and sea lions - CodyCross Answers Cheats and Solutions In one legend, seals, whales and other marine mammals were formed from her severed fingers. The Greeks associated them with both the sea and sun and were considered to be under the protection of the gods Poseidon and Apollo. Pinnipeds can be found in facilities around the world, as their large size and playfulness make them popular attractions. Zoologist Georges Cuvier noted during the 19th century that wild seals show considerable fondness for humans and stated that they are second only to some monkeys among wild animals in their easily tamability. Francis Galton noted in his landmark paper on domestication that seals were a spectacular example of an animal that would most likely never be domesticated despite their friendliness and desire for comfort due to the fact that they serve no practical use for humans. Some modern exhibits have rocky backgrounds with artificial haul-out sites and a pool, while others have pens with small rocky, elevated shelters where the animals can dive into their pools. More elaborate exhibits contain deep pools that can be viewed underwater with rock-mimicking cement as haul-out areas. The most common pinniped species kept in captivity is the California sea lion as it is both easy to train and adaptable. Other species popularly kept include the grey seal and harbor seal. Larger animals like walruses and Steller sea lions are much less common. -

List of Taxa for Which MIL Has Images
LIST OF 27 ORDERS, 163 FAMILIES, 887 GENERA, AND 2064 SPECIES IN MAMMAL IMAGES LIBRARY 31 JULY 2021 AFROSORICIDA (9 genera, 12 species) CHRYSOCHLORIDAE - golden moles 1. Amblysomus hottentotus - Hottentot Golden Mole 2. Chrysospalax villosus - Rough-haired Golden Mole 3. Eremitalpa granti - Grant’s Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus - Lowland Streaked Tenrec 3. Microgale cf. longicaudata - Lesser Long-tailed Shrew Tenrec 4. Microgale cowani - Cowan’s Shrew Tenrec 5. Microgale mergulus - Web-footed Tenrec 6. Nesogale cf. talazaci - Talazac’s Shrew Tenrec 7. Nesogale dobsoni - Dobson’s Shrew Tenrec 8. Setifer setosus - Greater Hedgehog Tenrec 9. Tenrec ecaudatus - Tailless Tenrec ARTIODACTYLA (127 genera, 308 species) ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BALAENIDAE - bowheads and right whales 1. Balaena mysticetus – Bowhead Whale 2. Eubalaena australis - Southern Right Whale 3. Eubalaena glacialis – North Atlantic Right Whale 4. Eubalaena japonica - North Pacific Right Whale BALAENOPTERIDAE -rorqual whales 1. Balaenoptera acutorostrata – Common Minke Whale 2. Balaenoptera borealis - Sei Whale 3. Balaenoptera brydei – Bryde’s Whale 4. Balaenoptera musculus - Blue Whale 5. Balaenoptera physalus - Fin Whale 6. Balaenoptera ricei - Rice’s Whale 7. Eschrichtius robustus - Gray Whale 8. Megaptera novaeangliae - Humpback Whale BOVIDAE (54 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Common Impala 3. Aepyceros petersi - Black-faced Impala 4. Alcelaphus caama - Red Hartebeest 5. Alcelaphus cokii - Kongoni (Coke’s Hartebeest) 6. Alcelaphus lelwel - Lelwel Hartebeest 7. Alcelaphus swaynei - Swayne’s Hartebeest 8. Ammelaphus australis - Southern Lesser Kudu 9. Ammelaphus imberbis - Northern Lesser Kudu 10. Ammodorcas clarkei - Dibatag 11. Ammotragus lervia - Aoudad (Barbary Sheep) 12. -

Global ICAP Workshop for Canids and Hyaenids Final Report.Pdf
Global Integrated Collection Assessment and Planning Workshop for Canids and Hyaenids Omaha, NE, US, 19 – 20 March 2016 Final Report Workshop organized by: AZA Canid and Hyaenid Taxon Advisory Group; EAZA Canid and Hyaenid Taxon Advisory Group; ZAA Carnivore Taxon Advisory Group; IUCN SSC Canid Specialist Group; IUCN SSC Hyaenid Specialist Group; and the IUCN SSC Conservation Planning Specialist Group (CPSG). Workshop financial support provided by: Saint Louis Zoo and a private donation Photo credits (front cover, left to right): Row 1: African wild dog (Yorkshire Wildlife Park); Swift fox (M. Sovada); Darwin fox education (M. Zordan); Fennec fox research (Fitbit; R. Meibaum); Row 2: Culpeo fox (Zoologico Nacional – Parque Metropolitano de Santiago, Chile); spotted hyena (Colchester Zoo); bush dog (M. Jacob); maned wolf (Temaiken Foundation); Row 3: Dhole (B. Gupta); Mexican wolf (J. Fallon); striped hyena (T. Rehse); black‐backed jackal (Amersfoort Zoo) A contribution of the IUCN SSC Conservation Planning Specialist Group IUCN encourages meetings, workshops and other fora for the consideration and analysis of issues related to conservation, and believes that reports of these meetings are most useful when broadly disseminated. The opinions and views expressed by the authors may not necessarily reflect the formal policies of IUCN, its Commissions, its Secretariat or its members. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. © Copyright CPSG 2018 Traylor‐Holzer, K., K. -

Canids of the World Wolves, Wild Dogs, Foxes
INTRODUCTION © Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. RECOGNITION The Canid family is a lineage of terrestrial carnivorans, adapted for swift running, which includes Wolves, Coyotes, Jackals, Foxes, Dogs, Dingoes, Dholes and other Dog-like mammals, with a total of 13 genera and at least 37 extant species. They are mostly social animals, living together in family units or small groups and behaving cooperatively. Most are seasonal breeders producing a single litter each year. They exhibit many reproductive and behavioral traits uncommon in other mammals, such as monogamy with paternal care, long-term incorporation of young adults into the social group, alloparenting, inhibition of reproduction in subordinate individuals, monoestrus, and a copulatory tie. They inhabit temperate and tropical forests, savanna, tundra and deserts throughout the world, with the exception of some oceanic islands and Antarctica. Most Canids feed on mammalian prey, but vegetable matter, carrion, and invertebrates are also an important source of food in many species. Size and body shape (fig. 1): Canids vary widely in size, from the Gray Wolf, which may be up to 160 cm long, and can weigh up to 80 kg, to the diminutive Fennec Fox, which may be as little as 24 cm in length, and weighs less than 1 kg. Most Fox species weigh 1.5 to 9.0 kg, while most other species are 5 to 27 kg. Body lengths (without tail) range between 35 and 160 cm, and tail lengths are approximately 12 to 56 cm. -

Functional Ecological Convergence Between the Thylacine and Small Prey-Focused Canids
Rovinsky et al. BMC Ecol Evo (2021) 21:58 BMC Ecology and Evolution https://doi.org/10.1186/s12862-021-01788-8 RESEARCH ARTICLE Open Access Functional ecological convergence between the thylacine and small prey-focused canids Douglass S. Rovinsky1* , Alistair R. Evans2,3 and Justin W. Adams1,3 Abstract Background: Morphological convergence is a fundamental aspect of evolution, allowing for inference of the biology and ecology of extinct species by comparison with the form and function of living species as analogues. The thylacine (Thylacinus cynocephalus), the iconic recently extinct marsupial, is considered a classic example of convergent evolu- tion with the distantly related placental wolf or dog, though almost nothing is actually known regarding its ecology. This lack of data leads to questions regarding the degree of convergence with, and the similarity of, the functional ecology of the thylacine and the wolf/dog. Here, we examined the cranium of the thylacine using 3D geometric morphometrics and two quantitative tests of convergence to more precisely determine convergent analogues, within a phylogenetically informed dataset of 56 comparative species across 12 families of marsupial and placental faunivo- rous mammals. Using this dataset, we investigated patterns of correlation between cranial shape and diet, phylogeny, and relative prey size across these terrestrial faunivores. Results: We fnd a correlation between cranial, facial, and neurocranial shape and the ratio of prey-to-predator body mass, though neurocranial shape may not correlate with prey size within marsupials. The thylacine was found to group with predators that routinely take prey smaller than 45% of their own body mass, not with predators that take subequal-sized or larger prey. -

Some Ecological Aspects of Dhole (Cuon Alpinus) in the Huai Kha Khaeng Wildlife Sanctuary, Uthai Thani Province, Thailand
FOLIA OECOLOGICA – vol. 46, no. 2 (2019), doi: 10.2478/foecol-2019-0012 Some ecological aspects of dhole (Cuon alpinus) in the Huai Kha Khaeng Wildlife Sanctuary, Uthai Thani Province, Thailand Khwanrutai Charaspet1, Ronglarp Sukmasuang1*, Noraset Khiowsree1, Nucharin Songsasen2, Saksit Simchareon3, Prateep Duengkae1 1Forest Biology Department, Kasetsart University, Phaholyothin Road, 10900, Bangkok, Thailand 2Smithsonian’s National Zoo & Conservation Biology Institute, 3001 Connecticut Avenue, NW, Washington, DC 20008, USA 3Department of National Parks Wildlife and Plant Conservation, Phaholyothin Road, 10900, Bangkok, Thailand Abstract Charaspet, K., Sukmasuang, R., Khiowsree, N., Songsasen, N., Simchareon, S., Duengkae, P., 2019. Some ecological aspects of dhole (Cuon alpinus) in the Huai Kha Khaeng Wildlife Sanctuary, Uthai Thani Province, Thailand. Folia Oecologica, 46: 91–100. The dhole (Cuon alpinus) is one of the least frequent studied endangered canid species and many aspects of ecological knowledge about this species are lacking. The objectives of this study were to investigate the spatial movement of dholes, prey abundance, prey selection, and prey overlaps with other large carnivorous species in the Huai Kha Khaeng Wildlife Sanctuary, Thailand, during November, 2017 and October, 2018. Two adult female dholes were captured and fitted with GPS collars. Twenty camera trap sets were systema- tically used to survey the area. Scat collection was conducted along forest roads and trails. The home range sizes and activity radii of the two dholes were 3,151.63 ha. and 1,442.84 m, and 33.39 ha and 331.56 m, re- spectively. The sambar deer (Rusa unicolor) was the most abundant prey species (30.93%). However, dhole fecal analysis showed that the monitored dholes preferred red muntjac (Muntiacus muntjak) (57.1%).