Temporal Changes in the Diet of Two Sympatric Carnivorous Mammals in a Protected Area of South–Central Chile Affected by a Mixed–Severity Forest Fire
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fur Trade and the Biotic Homogenization of Subpolar Ecosystems
Chapter 14 Fur Trade and the Biotic Homogenization of Subpolar Ecosystems Ramiro D. Crego, Ricardo Rozzi, and Jaime E. Jiménez Abstract At the southern end of the Americas exist one of the last pristine ecosys- tems in the world, the sub-Antarctic Magellanic forests ecoregion, protected by the Cape Horn Biosphere Reserve (CHBR). Despite its remote location, the CHBR has been subject to the growing infuences of globalization, a process that has driven cultural, biotic, and economic transformations in the region since the mid-twentieth century. One of the most important threats to these unique ecosystems is the increase of biological invasions. Motivated by the expanding fur industry that responded to the globalization process, American beavers (Castor canadensis), muskrats (Ondatra zibethicus), and American minks (Neovison vison) were introduced, inde- pendently, to the southern tip of South America. Research has shown that these three North American species have reassembled their native interactions to affect negatively the invaded ecosystems of the CHBR. Beavers affect river fow and R. D. Crego (*) Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA R. Rozzi Department of Philosophy and Religion and Department of Biological Sciences, University of North Texas, Denton, TX, USA Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad and Universidad de Magallanes, Punta Arenas, Chile J. E. Jiménez Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Department of Philosophy and Religion, University of North Texas, Denton, TX, USA Universidad de Magallanes, Punta Arenas, Chile © Springer Nature Switzerland AG 2018 233 R. -
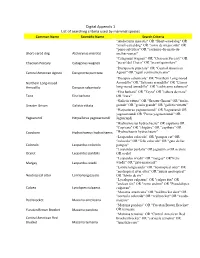
Digital Appendix 1 List of Searching Criteria Used by Mammal Species
Digital Appendix 1 List of searching criteria used by mammal species Common Name Scientific Name Search Criteria "Atelocynus microtis" OR "Short-eared dog" OR "small-eared dog" OR "zorro de oreja corta" OR "perro selvático" OR "cachorro-do-mato-de- Short-eared dog Atelocynus microtis orelhas-curtas" "Catagonus wagneri" OR "Chacoan Peccary" OR Chacoan Peccary Catagonus wagneri "pecarí del Chaco" OR "pecarí quimilero" “Dasyprocta punctate” OR "Central American Central American Agouti Dasyprocta punctata Agouti" OR "agutí centroamericano" “Dasypus sabanicola” OR "Northern Long-nosed Northern Long-nosed Armadillo" OR "Savanna armadillo" OR "Llanos Armadillo Dasypus sabanicola long-nosed armadillo" OR "cachicamo sabanero" “Eira barbara” OR "Tayra" OR "cabeza de mate" Taira Eira barbara OR "irara" “Galictis vittata” OR "Greater Grison" OR "furão- Greater Grison Galictis vittata grande" OR "grisón grande" OR "galictis vittatta" “Herpailurus yagouaroundi” OR Yaguarundi OR yagouaroundi OR "Puma yagouaroundi" OR Yaguarundi Herpailurus yagouaroundi Jaguarundi "Hydrochoerus hydrochaeris" OR capybara OR "Capivara" OR "chigüire" OR "capibara" OR Capybara Hydrochoerus hydrochaeris "Hydrochaeris hydrochaeris" “Leopardus colocolo” OR "pampas cat" OR "colocolo" OR "felis colocolo" OR "gato de las Colocolo Leopardus colocolo pampas" "Leopardus pardalis" OR jaguatirica OR ocelote Ocelot Leopardus pardalis OR ocelot “Leopardus wiedii” OR "margay" OR"Felis Margay Leopardus wiedii wiedii" OR "gato-maracajá" “Lontra longicaudis” OR "neotropical otter" OR "neotropical -

A Review of the Ecology of the Raccoon Dog (Nyctereutes Procyonoides) in Europe
A review of the ecology of the raccoon dog (Nyctereutes procyonoides) in Europe Jaap L. Mulder De Holle Bilt 17, NL-3732 HM De Bilt, the Netherlands, e-mail: [email protected] Abstract: The raccoon dog (Nyctereutes procyonoides) was introduced from East Asia into the former USSR between 1928 and 1957. Since then it has colonised a large part of Europe and is considered an invasive alien spe- cies. This paper reviews the current knowledge on the ecology of the raccoon dog in Europe, undertaken as a basis for a risk assessment. The raccoon dog is about the size of a red fox (Vulpes vulpes). In autumn it accumulates fat and, in areas with cold winters, it may stay underground for weeks. It does not dig and often uses badger (Meles meles) setts and fox earths for reproduction. Raccoon dogs are monogamous. Each pair occupies a fixed home range the periphery of which often overlaps with that of neighbours. Pre-breeding population density usually is between 0.5 and 1.0 adults/km2. Habitat use is characterised by a preference for shores, wet habitats and deciduous forests. Foraging raccoon dogs move quite slowly, mostly staying in cover. They are omnivorous gatherers rather than hunters. Their diet is variable, with amphibians, small mammals, carrion, maize and fruits being important components. There is no proof of a negative effect on their prey populations. Raccoon dogs produce a relatively large litter of usually 6 to 9 cubs. After six weeks the den is left and the whole family roams around. From July onwards the cubs, still only half grown, start to disperse. -

Vulpes Vulpes) Evolved Throughout History?
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Environmental Studies Undergraduate Student Theses Environmental Studies Program 2020 TO WHAT EXTENT HAS THE RELATIONSHIP BETWEEN HUMANS AND RED FOXES (VULPES VULPES) EVOLVED THROUGHOUT HISTORY? Abigail Misfeldt University of Nebraska-Lincoln Follow this and additional works at: https://digitalcommons.unl.edu/envstudtheses Part of the Environmental Education Commons, Natural Resources and Conservation Commons, and the Sustainability Commons Disclaimer: The following thesis was produced in the Environmental Studies Program as a student senior capstone project. Misfeldt, Abigail, "TO WHAT EXTENT HAS THE RELATIONSHIP BETWEEN HUMANS AND RED FOXES (VULPES VULPES) EVOLVED THROUGHOUT HISTORY?" (2020). Environmental Studies Undergraduate Student Theses. 283. https://digitalcommons.unl.edu/envstudtheses/283 This Article is brought to you for free and open access by the Environmental Studies Program at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Environmental Studies Undergraduate Student Theses by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. TO WHAT EXTENT HAS THE RELATIONSHIP BETWEEN HUMANS AND RED FOXES (VULPES VULPES) EVOLVED THROUGHOUT HISTORY? By Abigail Misfeldt A THESIS Presented to the Faculty of The University of Nebraska-Lincoln In Partial Fulfillment of Requirements For the Degree of Bachelor of Science Major: Environmental Studies Under the Supervision of Dr. David Gosselin Lincoln, Nebraska November 2020 Abstract Red foxes are one of the few creatures able to adapt to living alongside humans as we have evolved. All humans and wildlife have some id of relationship, be it a friendly one or one of mutual hatred, or simply a neutral one. Through a systematic research review of legends, books, and journal articles, I mapped how humans and foxes have evolved together. -

Allozyme Divergence Within the Canidae
Syst. Zooi, 36(4):339-355, 1987 ALLOZYME DIVERGENCE WITHIN THE CANIDAE ROBERT K. WAYNE1 AND STEPHEN J. O'BRIEN Laboratory of Viral Carcinogenesis, Section of Genetics, National Cancer Institute, Frederick, Maryland 21701 Abstract.—Protein products of 51 genetic loci were analyzed by gel electrophoresis using extracts of blood and tissue culture specimens from 12 of the 14 extant canid genera. Genetic Downloaded from distances were calculated and used to derive phenetic trees. The results suggest that the Canidae can be divided into several distinct groups. The wolf-like canids are a group that includes species in the genus Canis and Lycaon pictus (African wild dog). Speothos venaticus (Brazilian bush dog) is weakly associated with this group. Based on the calibration of a consensus tree with a fossil date, Canis mesomelas (black-backed jackal) and Speothos venaticus separated first, approximately 6 million years before present (MYBP). Lycaon pictus and C. latrans (coyote) separated from the line leading to C. lupus (grey wolf) and C. familiaris (domestic dog) approximately 3 MYBP. These http://sysbio.oxfordjournals.org/ results suggest that the blade-like trenchant heel on the carnassial tooth has evolved indepen- dently at least twice within the Canidae. Several distinct genetic stocks appear to have led to the extant South American canids. Chrys- ocyon brachyurus (maned wolf) is estimated to have diverged from Dusicyon vetulus (hoary fox) and Cerdocyon thous (crab-eating fox) approximately 6 MYBP. The divergence time of the last two genera is fairly recent (2-3 MYBP) and is coincident with the opening of the Panamanian land bridge. -

Chewing and Sucking Lice As Parasites of Iviammals and Birds
c.^,y ^r-^ 1 Ag84te DA Chewing and Sucking United States Lice as Parasites of Department of Agriculture IVIammals and Birds Agricultural Research Service Technical Bulletin Number 1849 July 1997 0 jc: United States Department of Agriculture Chewing and Sucking Agricultural Research Service Lice as Parasites of Technical Bulletin Number IVIammals and Birds 1849 July 1997 Manning A. Price and O.H. Graham U3DA, National Agrioultur«! Libmry NAL BIdg 10301 Baltimore Blvd Beltsvjlle, MD 20705-2351 Price (deceased) was professor of entomoiogy, Department of Ento- moiogy, Texas A&iVI University, College Station. Graham (retired) was research leader, USDA-ARS Screwworm Research Laboratory, Tuxtia Gutiérrez, Chiapas, Mexico. ABSTRACT Price, Manning A., and O.H. Graham. 1996. Chewing This publication reports research involving pesticides. It and Sucking Lice as Parasites of Mammals and Birds. does not recommend their use or imply that the uses U.S. Department of Agriculture, Technical Bulletin No. discussed here have been registered. All uses of pesti- 1849, 309 pp. cides must be registered by appropriate state or Federal agencies or both before they can be recommended. In all stages of their development, about 2,500 species of chewing lice are parasites of mammals or birds. While supplies last, single copies of this publication More than 500 species of blood-sucking lice attack may be obtained at no cost from Dr. O.H. Graham, only mammals. This publication emphasizes the most USDA-ARS, P.O. Box 969, Mission, TX 78572. Copies frequently seen genera and species of these lice, of this publication may be purchased from the National including geographic distribution, life history, habitats, Technical Information Service, 5285 Port Royal Road, ecology, host-parasite relationships, and economic Springfield, VA 22161. -

Activity Patterns in Sympatric Carnivores in the Nahuelbuta Mountain Range, Southern-Central Chile
Mammalia 2016; aop Alfredo H. Zúñiga*, Jaime E. Jiménez and Pablo Ramírez de Arellano Activity patterns in sympatric carnivores in the Nahuelbuta Mountain Range, southern-central Chile DOI 10.1515/mammalia-2015-0090 Received June 24, 2015; accepted August 26, 2016 Introduction Abstract: Species interactions determine the structure of Species interactions are one of the most studied topics in biological communities. In particular, interference behav- community ecology, as interspecific behavior can largely ior is critical as dominant species can displace subordi- determine the composition and structure of community nate species depending on local ecological conditions. In assemblages (Case and Gilpin 1974). For carnivores, inter- carnivores, the outcome of interference may have impor- specific interactions are particularly relevant because of tant consequences from the point of view of conservation, their role in top-down control in terrestrial ecosystems especially when vulnerable species are the ones suffering (Terborgh and Winter 1980). Nevertheless, given the key displacement. Using 24 baited camera traps and a sam- role of consumers and through trophic cascades, changes pling effort of 2821 trap nights, we examined the activ- in the environment could promote an increase of medium- ity patterns and spatial overlap of an assemblage of five sized carnivores or mesopredators, due to top predator sympatric carnivores in the Nahuelbuta Mountain Range, removal (Prange and Gehrt 2007) which can cause sub- in southern-central Chile. In this forested landscape we stantial changes in the dynamics of interaction among found predominantly nocturnal activity in all species, but sympatric species (Kamler et al. 2013), with adverse not for the puma (Puma concolor) and to a lesser extent, effects on subordinate species. -

The Ecological Effects of Providing Resource Subsidies to Predators
Global Ecology and Biogeography, (Global Ecol. Biogeogr.) (2014) bs_bs_banner RESEARCH The ecological effects of providing REVIEW resource subsidies to predators Thomas M. Newsome1,2*, Justin A. Dellinger3, Chris R. Pavey4, William J. Ripple2, Carolyn R. Shores3, Aaron J. Wirsing3 and Christopher R. Dickman1 1Desert Ecology Research Group, School of ABSTRACT Biological Sciences, University of Sydney, Aim Predators often have important roles in structuring ecosystems via their Sydney, NSW 2006, Australia, 2Trophic Cascades Program, Department of Forest effects on each other and on prey populations. However, these effects may be altered Ecosystems and Society, Oregon State in the presence of anthropogenic food resources, fuelling debate about whether the University, Corvallis, OR 97331, USA, 3School availability of such resources could alter the ecological role of predators. Here, we of Environmental and Forest Sciences, review the extent to which human-provided foods are utilised by terrestrial mam- University of Washington, Seattle, WA 98195, malian predators (> 1 kg) across the globe. We also assess whether these resources USA, 4CSIRO Land and Water Flagship, PO have a direct impact on the ecology and behaviour of predators and an indirect Box 2111, Alice Springs, NT 0871, Australia impact on other co-occurring species. Location Global. Methods Data were derived from searches of the published literature. To sum- marise the data we grouped studies based on the direct and indirect effects of resource subsidies on predators and co-occurring species. We then compared the types of predators accessing these resources by grouping species taxonomically and into the following categories: (1) domesticated species, (2) mesopredators and (3) top predators. -

Constraining the Time of Extinction of the South American Fox Dusicyon Avus (Carnivora, Canidae) During the Late Holocene
Geophysical Research Abstracts Vol. 12, EGU2010-577-1, 2010 EGU General Assembly 2010 © Author(s) 2009 Constraining the time of extinction of the South American fox Dusicyon avus (Carnivora, Canidae) during the late Holocene. Francisco Prevosti (1), Fernando Santiago (2), Luciano Prates (3), Mónica Salemme (2), and Fabiana Martin (4) (1) División Mastozoología, Museo Argentino de Ciencias Naturales, Angel Gallardo 470, Buenos Aires , Argentina ([email protected]), (2) CADIC, Ushuaia, Argentina, (3) División Arqueología, Museo de La Plata, La Plata, Argentina, (4) CEQUA, Punta Arenas, Chile The mass extinction at the end of the Pleistocene affected South America during the Late Pleistocene and the Early Holocene, when megamammals and large mammals disappeared. Several carnivores became extinct, like the sabretooth Smilodon, the short face bear (Arctotherium) and some large canids (i.e. Protocyon, Canis dirus). After this mass event virtually no carnivores became extinct in South America. The only exception is the fox Dusicyon avus, a middle sized canid (estimated body mass between 10-15 kg) with a more carnivore diet than the living South American foxes (i.e. Lycalopex culpaeus). The last record of the species comes from middle-late Holocene archaeological sites in the Pampean Region (Argentina) and Patagonia (Argentina and Chile). During the Late Pleistocene D. avus had a wide distribution, that covered part of Uruguay, Argentina (Buenos Aires province) and the southernmost Chile. Albeit some remains from late Holocene sites have been published, these remains lack of isotopic dates that could (allow?) constraint (to determine) the date of extinction of this fox. In this contribution we present several new records from the Pampean Region and Patagonia, and several taxon dates. -

Kansas Cooperative Fish and Wildlife Research Unit 25
KANSAS COOPERATIVE FISH AND WILDLIFE RESEARCH UNIT 25 YEARS (1991-2016) OF COOPERATIVE RESEARCH COMPILED BY: JOYCE BRITE JULY 2016 Kansas State University Preface The Kansas Cooperative Fish and Wildlife Research Unit is jointly sponsored and financed by the U.S. Geological Survey-Biological Resources Division, Kansas Department of Wildlife, Parks, and Tourism, Kansas State University, U.S. Fish and Wildlife Service, and the Wildlife Management Institute. In 1960, Congress gave statutory recognition to the Cooperative Research Unit program by enactment of Public Law 86-686. The act reads: "To facilitate cooperation between the Federal Government, colleges and universities, the States, and private organizations for cooperative unit programs of research and education relating to fish and wildlife, and for other purposes. Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled, That, for the purpose of developing adequate, coordinated, cooperative research and training programs for fish and wildlife resources, the Secretary of the Interior is authorized to continue to enter into cooperative agreements with colleges and universities, with game and fish departments of the several States, and with nonprofit organizations relating to cooperative research units: Provided, That Federal participation in the conduct of such cooperative unit programs shall be limited to the assignment of the Department of the Interior technical personnel by the Secretary to serve at the respective units, to supply for the use of the particular unit's operations such equipment as may be available to the Secretary for such purposes, and the payment of incidental expenses of Federal personnel and employees of cooperating agencies assigned to the units. -

Report of the Presence of Wild Animals
Report of the Presence of Wild Animals The information recorded here is essential to emergency services personnel so that they may protect themselves and your neighbors, provide for the safety of your animals, ensure the maximum protection and preservation of your property, and provide you with emergency services without unnecessary delay. Every person in New York State, who owns, possesses, or harbors a wild animal, as set forth in General Municipal Law §209-cc, must file this Report annually, on or before April 1, of each year, with the clerk of the city, village or town (if outside a village) where the animal is kept. A list of the common names of animals to be reported is enclosed with this form. Failure to file as required will subject you to penalties under law. A separate Report is required to be filed annually for each address where a wild animal is harbored. Exemptions: Pet dealers, as defined in section 752-a of the General Business Law, zoological facilities and other exhibitors licensed pursuant to U.S. Code Title 7 Chapter 54 Sections 2132, 2133 and 2134, and licensed veterinarians in temporary possession of dangerous dogs, are not required to file this report. Instructions for completing this form: 1. Please print or type all information, using blue or black ink. 2. Fill in the information requested on this page. 3. On the continuation sheets, fill in the information requested for each type of animal that you possess. 4. Return the completed forms to the city, town, or village clerk of each municipality where the animal or animals are owned, possessed or harbored. -

How Are Wetlands and Biological Interactions Related to Carnivore Distributions at High Altitude?
Journal of Arid Environments 115 (2015) 14e18 Contents lists available at ScienceDirect Journal of Arid Environments journal homepage: www.elsevier.com/locate/jaridenv Short communication How are wetlands and biological interactions related to carnivore distributions at high altitude? * G.A.E. Cuyckens a, b, , P.G. Perovic c, L. Cristobal d a Consejo Nacional de Investigaciones Científicas y Tecnicas (CONICET), Argentina b Centro de Estudios Territoriales, Ambientales y Sociales (CETAS), Facultad de Ciencias Agrarias, Universidad Nacional de Jujuy, Argentina c Administracion de Parques Nacionales, Delegacion Noroeste, Argentina d Fundacion ProYungas, Argentina article info abstract Article history: Determining the geographic range of species is a main objective in ecology and has implications for Received 20 December 2013 conservation. Key determinants of carnivore distribution in dry environments are competition and the Received in revised form availability of water. Here, we gathered and mapped the available information on carnivore habitat 18 December 2014 quality in the high Andes and Puna in the extreme north of Argentina. We investigated four carnivore Accepted 23 December 2014 species: the Andean cat (Leopardus jacobita), the Pampas cat (Leopardus colocolo), the cougar (Puma Available online concolor) and the culpeo fox (Lycalopex culpaeus). We assessed the main determinants of their distri- bution, testing explicitly for the effects of seasonal and temporal wetlands and biological interactions. We Keywords: Competition used species distribution models, and created biophysical models using environmental and landscape High Andes variables. Then, by including the four species' biophysical models into the model of the focal species, we Leopardus jacobita tested for the importance of biological interactions. Wetlands were most important for the culpeo fox, L.