Catalytic Radical-Polar Crossover Ritter Reaction Eric E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 10 Carbon-Nitrogen Bonds Formation I
NPTEL – Chemistry – Principles of Organic Synthesis Lecture 10 Carbon-Nitrogen Bonds Formation I 4.1 Principles The methods for the formation of bonds between nitrogen and aliphatic carbon can be broadly divided into two categories: (i) reaction of nucleophilic nitrogen with electrophilic carbon, and (ii) reaction of electrophilic nitrogen with nucleophilic carbon. In this section, we will try to cover some of the important reactions. 4.2 Substitution of Nitrogen Nucleophiles at Saturated Carbon 4.2.1 Ritter Reaction Treatment of a tertiary alcohol or the corresponding alkene with concentrated sulphuric acid and a nitrile affords an amide that in acidic conditions undergoes hydrolysis to give amine. First, a R H, RCN H3O + RCO H OH N O NH 2 H2O H 2 Mechanism OH2 H -H2O :NC-R H2O OH OH N N R N R 2 C R OH O H2O proton H3O + RCO2H N R N R NH2 -H3O transfer H Scheme 1 tertiary carbocation is formed which is attacked by the nitrile to give a quaternary ion. The latter is decomposed by water to provide an amide which, in acidic conditions, is hydrolyzed to give an amine (Scheme 1). Joint initiative of IITs and IISc – Funded by MHRD Page 1 of 35 NPTEL – Chemistry – Principles of Organic Synthesis Examples: Me H N H2SO4 + N Chlorobenzene O 91% S. J. Chang, Organic Process Research and Development 1999, 3, 232. Me Me Me Me Me Me Me Me SnCl4 MeCN CH2 CH2 Cl N N T.-L. Ho, R.-J. Chein, J. Org. Chem. 2004, 69, 591. Me Me O Ph HNCHO Ph OH Ph HN H2SO4 Me H2SO4 N TMSCN N MeCN N Bn Bn Bn H. -

MDA) Analogs and Homologs
Terry A. Dal Cason, 1 M.Sc. An Evaluation of the Potential for Clandestine Manufacture of 3,4-Methylenedioxyamphetamine (MDA) Analogs and Homologs REFERENCE: Dal Cason, T. A., "An Evaluation of the Potential for Clandestine Manu- facture of 3,4-Methylenedioxyamphetamine (MDA) Analogs and Homologs," Journal of Forensic Sciences, JFSCA, Vol. 35, No.3, May 1990, pp. 675-697. ABSTRACT: Encountering a novel controlled-substance analog (designer drug) has become a distinct possibility for all forensic drug laboratories. 3,4-Methylenedioxyamphetamine (MDA) in particular is a receptive parent compound for the molecular modifications which produce such homo logs and analogs. The identification of these compounds, however, can prove to be an arduous task. It would be desirable to direct the focus of the identification to those compounds which are the more likely candidates for clandestine-laboratory synthesis. The process of narrowing the range of theoretical possibilities to logical choices may be enhanced by using a suitable predictive scheme. Such a predictive scheme for MDA analogs is presented based on putative Central Nervous System activity, existence or formulation of a reasonable synthesis method, and availability of the required precursors. KEYWORDS: toxicology, 3,4-methylenedioxyamphetamine, drug identification To circumvent statutes enacted to control the use of various dangerous drugs (controlled substances), clandestine laboratory operators will sometimes make minor alterations in the molecular structure of a parent compound. These structural changes are reflected in the chemical nomenclature of the new analog or homolog, and, in the past/ had effectively removed it from the purview of the law. Such modifications, at least in the case of 3,4- methylenedioxyamphetamine (MDA), do not appear to be haphazard. -
Alcohols and Ethers
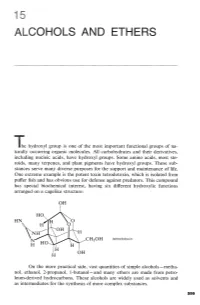
ALCOHOLS AND ETHERS The hydroxyl group is one of the most important functional groups of na- turally occurring organic molecules. All carbohydrates and their derivatives, including nucleic acids, have hydroxyl groups. Some amino acids, most ste- roids, many terpenes, and plant pigments have hydroxyl groups. These sub- stances serve many diverse purposes for the support and maintenance of life. One extreme example is the potent toxin tetrodotoxin, which is isolated from puffer fish and has obvious use for defense against predators. This compound has special biochemical interest, having six different hydroxylic functions arranged on a cagelike structure: tetrodotoxin On the more practical side, vast quantities of simple alcohols - metha- nol, ethanol, 2-propanol, 1-butan01 - and many ethers are made from petro- leum-derived hydrocarbons. These alcohols are widely used as solvents and as intermediates for the synthesis of more complex substances. 15 Alcohols and Ethers The reactions involving the hydrogens of alcoholic OH groups are expected to be similar to those of water, HOH, the simplest hydroxylic com- pound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the 8-H bond but also with processes that result in cleavage of the C-0 bond, or changes in the organic group R. The simple ethers, ROR, do not have 0-H bonds, and most of their reactions are limited to the substituent groups. The chemistry of ethers, there- fore, is less varied than that of alcohols. This fact is turned to advantage in the widespread use of ethers as solvents for a variety of organic reactions, as we already have seen for Grignard reagents (Section 14- 10). -

Amidation of Alcohols with Nitriles Under Solvent-Free Conditions
Journal of Oleo Science Copyright ©2010 by Japan Oil Chemists’ Society J. Oleo Sci. 59, (11) 607-613 (2010) Amidation of Alcohols with Nitriles under Solvent-free Conditions Using Molecular Iodine as a Catalyst Yoshio Kasashima1* , Atsushi Uzawa1, Kahoko Hashimoto2, Yu Yokoyama3, Takashi Mino3, Masami Sakamoto3 and Tsutomu Fujita3 1 Education Center, Faculty of Engineering, Chiba Institute of Technology (2-1-1shibazono, Narashino-shi, Chiba 275-0023, JAPAN) 2 Department of Life Environmental Sciences, Faculty of Engineering, Chiba Institute of Technology (2-17-1 Tsudanuma, Narashino-shi, Chiba 275-0016, JAPAN) 3 Graduate School of Engineering, Chiba University (1-33 Yayoi-cho, Inage-ku, Chiba-shi, Chiba 263-8522, JAPAN) Abstract: The reactions of alcohols with nitriles under solvent-free conditions, using molecular iodine as a catalyst, were investigated. The reaction of 1-phenylethanol with propanenitrile produced the amide N-(1- phenylethyl)propanamide, by dehydration and tautomerization, in 71% yield, under the following conditions: temperature=90℃, alcohol:iodine molar ratio=1:0.2, alcohol:nitrile molar ratio=1:5, and reaction time=5 h. The amidation reactivity depended on the stability of the cationic intermediate formed from the alcohol. The reaction of (−)-borneol with benzonitrile produced a racemic amide in 83% yield. Key words: iodine, solvent-free, amidation, borneol 1 INTRODUCTION spectrometer(JASCO, Easton, MD, USA). Gas chromatog- Compounds with amide linkages are important in the raphy/mass spectra(GC-MS)were recorded on a Shimadzu chemical industry. The Ritter reaction, which is the reac- GCMS-QP5050A(Shimadzu, Kyoto, Japan)[column: JW tion of alcohols with nitriles, is one method of producing Scientifi c DB-5ms(30 m×0.25 mm, 0.25 μm fi lm), Agilent, amides1-5). -

Eur. J. Org. Chem. 12 (2015) 2727-2732; Doi: 10.1002/Ejoc.201500051
Eur. J. Org. Chem. 12 (2015) 2727-2732; doi: 10.1002/ejoc.201500051. Mechanochemical Ritter reaction: a rapid approach to functionalized amides at room temperature Irena Dokli and Matija Gredičak*[a] mostly prepared by standard acid chloride-amine couplings or by recently developed ortho-directed functionalizations,[13] metal- catalysed amidations[14] and metal-free carboxyamidations.[15] Abstract: A fast and efficient mechanochemical Ritter reaction In this paper, we report a general procedure for Brønsted acid between alcohols and nitriles under mild conditions is demonstrated. catalysed mechanochemical Ritter reaction under mild The reaction proceeds rapidly at room temperature in a solvent-free conditions: room temperature, short reaction time, and a solvent- or low-solvent environment, utilizing a Brønsted acid catalyst. Its free or low-solvent environment. The versatility of the protocol is general application has been verified through a substrate screening veryfied through a wide substrate scope investigation, including investigation comprising a wide range of functionalized nitriles, as functionalized nitriles, as well as secondary and tertiary alcohols. well as secondary and tertiary alcohols. Mechanochemistry has been recognized as one of the most successful modes of solvent-free synthesis.[16] Introduction Mechanochemical reactions, usually performed in ball mills, are now present in all fields of chemistry, and their application in [17] The Ritter reaction is an organic reaction that allows formation of organic synthesis is increasing. Recently, it has been shown amides from a carbocation precursor (tertiary alcohol or that conditions produced by a ball mill could be compared to substituted olefin) and a nitrile using a strong acid catalyst.[1] those produced when performing the same reaction at elevated Although the Ritter reaction found its application in drug,[2] and temperature in a solution, though the temperature in the vial [18] natural product and natural product-like syntheses,[3] the remains virtually ambient. -

Preparation of Different Amides Via Ritter Reaction from Alcohols
J. Chem. Sci. Vol. 124, No. 5, September 2012, pp. 1025–1032. c Indian Academy of Sciences. Preparation of different amides via Ritter reaction from alcohols and nitriles in the presence of silica-bonded N- propyl sulphamic acid (SBNPSA) under solvent-free conditions MARYAM-SADAT SHAKERIa,∗, HASSAN TAJIKa,b and KHODABAKHSH NIKNAMa aDepartment of Chemistry, Faculty of Sciences, Persian Gulf University, Bushehr 75169, Iran bDepartment of Chemistry, Faculty of Sciences, Guilan University, Rasht, Iran e-mail: [email protected] MS received 29 November 2011; revised 22 May 2012; accepted 18 June 2012 Abstract. A number of methods have been proposed for the modification of the Ritter reaction. However, many of these methods involve the use of strongly acidic conditions, stoichiometric amounts of reagents, harsh reaction conditions and extended reaction times. Therefore, the development of mild, efficient, convenient and benign reagents for the Ritter reaction is desirable. In this research, we have developed a clean and environmen- tally friendly protocol for the synthesis of amides by using different benzylic or tertiary alcohols and different nitriles in the presence of silica-bonded N- propyl sulphamic acid (SBNPSA) as catalyst under solvent-free conditions in high yields. Keywords. Ritter reaction; SBNPSA; alcohol; nitrile; amide. 1. Introduction be reused. In recent years, the search for environmen- tally benign chemical processes or methodologies has The conversion of nitriles to amides by reaction with received much attention. Heterogenization of homoge- alcohols or alkenes in the presence of sulphuric acid neous catalysts has been an interesting area of research is named Ritter reaction. Acidification of the appro- from an industrial point of view; this combines the priate alcohol or alkene generates a carbenium ion advantages of homogeneous catalysts (high activity, which reacts with nitrile. -

Synthesis and Evaluation of Cocaine Analogs for Activity As Cocaine Antagonists and Synthesis of Novel Prodrugs with Antiarthritic Potential
Medical University of South Carolina MEDICA MUSC Theses and Dissertations 1996 Synthesis and Evaluation of Cocaine Analogs for Activity as Cocaine Antagonists and Synthesis of Novel Prodrugs with Antiarthritic Potential Bonnie Lisa Barr Medical University of South Carolina Follow this and additional works at: https://medica-musc.researchcommons.org/theses Recommended Citation Barr, Bonnie Lisa, "Synthesis and Evaluation of Cocaine Analogs for Activity as Cocaine Antagonists and Synthesis of Novel Prodrugs with Antiarthritic Potential" (1996). MUSC Theses and Dissertations. 92. https://medica-musc.researchcommons.org/theses/92 This Dissertation is brought to you for free and open access by MEDICA. It has been accepted for inclusion in MUSC Theses and Dissertations by an authorized administrator of MEDICA. For more information, please contact [email protected]. SYNTHESIS AND EVALUATION OF COCAINE ANALOGS FOR ACTIVITY AS COCAINE ANTAGONISTS AND SYNTHESIS OF NOVEL PRODRUGS WITH ANTIARTHRITIC POTENTIAL by Bonnie Lisa Barr A dissertation submitted to the faculty of the Medical University of South Carolina in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Graduate Studies. Department of Pharmaceutical Sciences 1996 Approved by: TABLE OF CONTENTS Abstract VI Acknowledgements IX I. Background and Significance 1 A. Salicylic Acid Derivatives 7 B. 5-Pyrazolones 9 C. Indomethacin 10 D. Sulindac 12 E. Diclofenac 12 F. Etodolac 14 G. Tolmetin 14 H. Phenyl-alpha-methyl acetic acids 15 I. Other Carboxylic Acids and Derivatives 16 J. N -arylanthranilic Acids 19 K.Oxicams 19 L. Disease Modifying Antirheumatic Drugs 21 1. 4-Aminoquinoloines 21 2. Gold Compounds 22 ii 3. -

Catalytic Approaches to the Synthesis of Amide Bonds
Catalytic Approaches to the Synthesis of Amide Bonds C. Liana Allen A thesis submitted for the degree of Doctor of Philosophy University of Bath Department of Chemistry February 2012 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. A copy of this thesis has been supplied on the condition that anyone who consults it is understood to recognise that its copyright rests with its author and they must not copy it or use material from it except as permitted by law or with the consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. C. Liana Allen CONTENTS Acknowledgments iv Abbreviations v Abstract vii 1. Introduction 1 1.1 Introduction to Amides 1 1.2 Synthesis of Amides 4 1.2.1 Direct Coupling Methods 4 1.2.2 Coupling Reagents 7 1.2.3 Enzymatic Methods 14 1.2.4 Non-Metal Catalysts 16 1.2.5 Metal Catalysts 20 1.3 Summary 44 1.4 Project Aims 44 2. Results and Discussion I – Amides from Nitriles 45 2.1 Introduction 46 2.2 Initial Work 46 2.3 Optimisation 47 2.4 Mechanism Studies 53 2.5 Further Work 54 2.6 Conclusions 56 3. Results and Discussion II – Amides from Oximes 57 3.1 Introduction 58 3.2 Initial Work 58 3.3 Primary Amides 59 3.4 Secondary Amides 70 3.5 Mechanism Studies 79 3.5.1 Primary Amide Formation 79 3.5.2 Secondary Amide Formation 89 3.6 Conclusions 94 3.7 Future Work 95 4. -

A Thesis Presented to the Faculty of Alfred University Reactions
A Thesis Presented to The Faculty of Alfred University Reactions Mediated by the Conducting Polymer Poly-(3,4-ethylenedioxythiophene) (PEDOT) Danica Ostrander In Partial Fulfillment of the Requirements for The Alfred University Honors Program Date: May 14, 2013 Under the Supervision of: Chair: Dr. John G. D’Angelo Committee Members: Dr. Jeremy Cody Dr. Geoffrey Bowers TABLE OF CONTENTS INTRODUCTION 3-13 The Ritter Reaction 3-6 General Uses of Conducting Polymers 6 Reactions of Conducting Polymers 7 Poly-(3,4-ethylenedioxy thiophene) (PEDOT) 7-9 The Ritter Reaction and PEDOT 9-13 RESULTS 14-26 PEDOT-Mediated Ritter Reactions with Various Alcohols 14-17 Stoichiometric Attempts 18-20 PEDOT-Mediated Ritter Reactions with Various Nitriles 20-21 Mechanisitic Studies 21-24 Other Reactions Mediated by PEDOT 24-26 EXPERIMENTAL 27-30 CONCLUSIONS AND FUTURE WORK 31-32 APPENDIX A: LIST OF COMPOUNDS 33-36 REFERENCES 37-38 2 INTRODUCTION The Ritter Reaction: The reaction involving formation of n-carboxamides (1) from aliphatic or aromatic nitriles (2) and carbocations (3) is known as the Ritter reaction and was first reported by J. J. Ritter and P. P. Minieri in 1948 (Scheme 1).1 Scheme 1: General Ritter Reaction The Ritter reaction has been widely used for the preparation of acyclic amides as well as heterocycles, including lactams (4), oxazolines (5), and dihydroisoquinolines (6).1 In addition, the reaction has been used in the synthesis of certain natural product derivatives, such as in the completion of the first total synthesis of (-)-4-epiaxinyssamine (7), an epimer of axinyssamine (8), the latter of which shows cytotoxic acitivty against tumor cell lines. -

Oxidative Desulfurization of Azole-2-Thiones with Benzoyl Peroxide
OXIDATIVE DESULFURIZATION OF AZOLE-2-THIONES WITH BENZOYL PEROXIDE by DEREK MICHAEL WOLFE (Under the Direction of Peter R. Schreiner) ABSTRACT 1-Alkyl-3-methylimidazole-2-thiones were prepared in one pot and subsequently converted to 1-alkyl-3-methylimidazolium benzoates through a sequence consisting of an oxidative desulfurization with benzoyl peroxide and a novel anion exchange. Also reported are the outcomes of exchanges with other anions, acidifications of the imidazolium benzoates to other salts, and the syntheses of both 1,3-diphenylimidazolium and 3-alkylthiazolium salts from the corresponding azole-2-thiones. This oxidative desulfurization was also appropriate for the synthesis of neutral imidazoles from 1-alkylimidazole-2-thiones, which were prepared from amino acids by way of 2-thiohydantoins. Four such sequences are described, one of which constitutes a formal synthesis of three imidazole alkaloids from the sponge Leucetta. The merits of these routes in terms of both adaptability and operational simplicity are emphasized. A chiral imidazolium salt featuring camphor was pursued, but an imidazole-2-thione precursor suited to desulfurization could not be prepared; the desired salt could not be assembled with conventional methods, either. The unsuitability of some imidazolium ionic liquids in an adaptation of the phase transfer catalyzed halogenation is discussed in the context of γ-adamantane amino acids. INDEX WORDS: Anion exchange, Desulfurizations, Heterocycles, Imidazoles, Ionic Liquids, Oxidations, Sulfur OXIDATIVE DESULFURIZATION OF AZOLE-2-THIONES WITH BENZOYL PEROXIDE by DEREK MICHAEL WOLFE B.S., Roanoke College, 1999 A Dissertation Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY ATHENS, GEORGIA 2007 © 2007 Derek M. -

Benzyl Amide Synthesis Via
AN INVESTIGATION OF N-BENZYL AMIDE SYNTHESIS VIA OXYPYRIDINIUM SALTS A THESIS SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE MASTER OF SCIENCE BY MARYAM BANIASAD DR. PHILIP ALBINIAK – ADVISOR BALL STATE UNIVERSITY MUNCIE, INDIANA JULY 2017 i Acknowledgements I would like to express my deepest gratitude for my advisor, Dr. Albiniak for his patience, motivation, and support. Dr. Albiniak was always willing to help me improve in the lab. His advices in the preparation of this thesis is thankfully appreciated. In addition, I would also like to express my appreciation to my committee members, Dr. Sammelson and Dr. Rayat, for their help throughout the entire process. I could not have accomplished this goal without their significant contributions. I gratefully acknowledge my friends, lab mates, classmates, and faculties at the Department of Chemistry, Ball State University. I would like to extend my special thanks to my beloved family, whom I owe my whole life and success. Thank you Mom, Dad, and Salar for your love and support. Distance cannot stop me from loving you. Lastly, my special thanks go to Sajad, for his continued support throughout this program. ii Table of Contents Chapter 1: Background and Introduction Section 1.1: Amides………………………………………………………….……………………1 Section 1.2: Applications of Amides ……………………..………………………………………2 Section 1.3: Synthesis of Amides …………………...……………………………………………4 Section 1.3.1: Schotten-Baumann Reaction………………………………………………………5 Section 1.3.2: Lumière–Barbier Reaction………………………………………………………...6 -

New Stereoselective Reactions to Form Amido Alkyl C-N and Vinyl Triflate C-O
NEW STEREOSELECTIVE REACTIONS TO FORM AMIDO ALKYL C-N AND VINYL TRIFLATE C-O BONDS VIA CARBOCATION INTERMEDIATES & ULTRAFAST SILICON FLUORINATION METHODOLOGIES FOR APPLICATIONS IN PET IMAGING by Mohammed Alhuniti A Dissertation Submitted to the Faculty of The Charles E. Schmidt College of Science in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Florida Atlantic University Boca Raton, Florida December 2014 ACKNOWLEDGEMENTS I would like to thank my advisor, Prof. Salvatore D. Lepore, for his excellent guidance throughout my doctoral studies. Prof. Lepore is truly an outstanding mentor who devotes his time to teach more than organic chemistry. I want to thank my committee members Professors William Roush, Stéphane Roche, and Lyndon West for their time and insightful advice on my research. I thank Susan Lepore for her efforts to facilitate my research in the Lepore group. I also thank all former and current members of the group. Finally, I wish to express my gratitude to my family who have continually supported me. I sincerely appreciate the endless encouragement of my mother, my brothers, and my lovely wife and daughters. iv ABSTRACT Author: Mohammed Alhuniti Title: New Stereoselective Reactions to Form Amido Alkyl C-N and Vinyl Triflate C-O Bonds via Carbocation Intermediates & Ultrafast Silicon Fluorination Methodologies for Applications in PET Imaging. Institution: Florida Atlantic University Thesis Advisor: Dr. Salvatore D. Lepore Degree: Doctor of Philosophy Year: 2014 We report here the development of a Lewis acid catalyzed method for the dehydrative coupling of cyclic alcohols and nitriles to form amides with retention of configuration.