Oxidative Desulfurization of Azole-2-Thiones with Benzoyl Peroxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanistic Insight to the Chemical Treatments of Monolayer Transition Metal Disulfides for Photoluminescence Enhancement
Mechanistic Insight to the Chemical Treatments of Monolayer Transition Metal Disulfides for Photoluminescence Enhancement Zhaojun Li1,2, Hope Bretscher1, Yunwei Zhang1, Géraud Delport1, James Xiao1, Alpha Lee1, Samuel D. Stranks1,3, and Akshay Rao1* 1Cavendish Laboratory, University of Cambridge, JJ Thomson Avenue, CB3 0HE, Cambridge, United Kingdom E-mail: [email protected] 2Molecular and Condensed Matter Physics, Department of Physics and Astronomy, Uppsala University, 75120 Uppsala, Sweden 3Department of Chemical Engineering & Biotechnology, University of Cambridge, Philippa Fawcett Drive, CB3 0AS, Cambridge, United Kingdom Keywords: chemical treatments, transition metal disulfides, photoluminescence, trion suppression, p- doping Abstract There is a growing interest in obtaining high quality monolayer transition metal disulfides (TMDSs) for optoelectronic device applications. Surface chemical treatments using a range of chemicals on monolayer TMDSs have proven effective to improve their photoluminescence (PL) yield. However, the underlying mechanism for PL enhancement by these treatments is not clear, which prevents a rational design of passivation strategies. In this work, a simple and effective approach to significantly enhance PL of TMDSs is demonstrated by using a family of cation donors, which we show to be much more effective than commonly used p-dopants which achieve PL enhancement through electron transfer. We develop a detailed mechanistic picture for the action of these cation donors and demonstrate that one of them, Li-TFSI (bistriflimide), enhances the PL of both MoS2 and WS2 to a level double that compared to the widely discussed and currently best performing “super acid” H-TFSI treatment. In addition, the ionic salts used in chemical treatments are compatible with a range of greener solvents and are easier to handle than super-acids, which provides the possibility of directly treating TMDSs during device fabrication. -

Lecture 10 Carbon-Nitrogen Bonds Formation I
NPTEL – Chemistry – Principles of Organic Synthesis Lecture 10 Carbon-Nitrogen Bonds Formation I 4.1 Principles The methods for the formation of bonds between nitrogen and aliphatic carbon can be broadly divided into two categories: (i) reaction of nucleophilic nitrogen with electrophilic carbon, and (ii) reaction of electrophilic nitrogen with nucleophilic carbon. In this section, we will try to cover some of the important reactions. 4.2 Substitution of Nitrogen Nucleophiles at Saturated Carbon 4.2.1 Ritter Reaction Treatment of a tertiary alcohol or the corresponding alkene with concentrated sulphuric acid and a nitrile affords an amide that in acidic conditions undergoes hydrolysis to give amine. First, a R H, RCN H3O + RCO H OH N O NH 2 H2O H 2 Mechanism OH2 H -H2O :NC-R H2O OH OH N N R N R 2 C R OH O H2O proton H3O + RCO2H N R N R NH2 -H3O transfer H Scheme 1 tertiary carbocation is formed which is attacked by the nitrile to give a quaternary ion. The latter is decomposed by water to provide an amide which, in acidic conditions, is hydrolyzed to give an amine (Scheme 1). Joint initiative of IITs and IISc – Funded by MHRD Page 1 of 35 NPTEL – Chemistry – Principles of Organic Synthesis Examples: Me H N H2SO4 + N Chlorobenzene O 91% S. J. Chang, Organic Process Research and Development 1999, 3, 232. Me Me Me Me Me Me Me Me SnCl4 MeCN CH2 CH2 Cl N N T.-L. Ho, R.-J. Chein, J. Org. Chem. 2004, 69, 591. Me Me O Ph HNCHO Ph OH Ph HN H2SO4 Me H2SO4 N TMSCN N MeCN N Bn Bn Bn H. -

Base Stable and Basic Ionic Liquids for Catalysis
DOCTOR OF PHILOSOPHY Base stable and basic ionic liquids for catalysis McNeice, Peter Award date: 2020 Awarding institution: Queen's University Belfast Link to publication Terms of use All those accessing thesis content in Queen’s University Belfast Research Portal are subject to the following terms and conditions of use • Copyright is subject to the Copyright, Designs and Patent Act 1988, or as modified by any successor legislation • Copyright and moral rights for thesis content are retained by the author and/or other copyright owners • A copy of a thesis may be downloaded for personal non-commercial research/study without the need for permission or charge • Distribution or reproduction of thesis content in any format is not permitted without the permission of the copyright holder • When citing this work, full bibliographic details should be supplied, including the author, title, awarding institution and date of thesis Take down policy A thesis can be removed from the Research Portal if there has been a breach of copyright, or a similarly robust reason. If you believe this document breaches copyright, or there is sufficient cause to take down, please contact us, citing details. Email: [email protected] Supplementary materials Where possible, we endeavour to provide supplementary materials to theses. This may include video, audio and other types of files. We endeavour to capture all content and upload as part of the Pure record for each thesis. Note, it may not be possible in all instances to convert analogue formats to usable digital formats for some supplementary materials. We exercise best efforts on our behalf and, in such instances, encourage the individual to consult the physical thesis for further information. -

Formation of Complexes in RTIL and Ion Separations
20 Formation of Complexes in RTIL and Ion Separations Konstantin Popov1,2, Andrei Vendilo2, Igor Pletnev3, Marja Lajunen4, Hannu Rönkkömäki5 and Lauri H.J. Lajunen4 1Department of Physical and Colloid Chemistry, Moscow State University of Food Production, Moscow, 2Institute of Reagents and high purity Substances (IREA), Moscow, 3Department of Chemistry M.V.Lomonosov State University , Moscow, 4Department of Chemistry, Oulu University, Oulu, 5Finnish institute of Occupational Health, Oulu, 1,2,3Russia 4,5Finland 1. Introduction Room temperature ionic liquids (RTILs) are gaining an increasing interest as a unique medium for a complex formation and development of new inorganic materials (Cocalia et al, 2006; Billard et al, 2003; Yan et al, 2010; Vendilo et al, 2010; Nockemann et al, 2009; Nockemann et al, 2008; Murding & Tang, 2010; Billard & Gaillard, 2009; Taubert, 2004; Tang et al, 2008). Among the most promising fields the RTIL-based lithium batteries (Lewandowski & Swiderska- Mocek, 2009; Rosol et al, 2009) and recent applications of ionic liquids in the separation technology (Dundan & Kyung, 2010; Dietz, 2006) can be considered as a “hot” research topic. The present review is therefore focused on the role of cation complexes in RTIL-based metal ion separations, while the other important aspects of inorganic salt behaviour in RTILs are excellently summarised in another chapter of this book (Nockemann, 2011). RTILs are intensively studied in solvent extraction processes due to such important advantages over conventional organic diluents as negligible vapor pressure, low flammability, moisture stability, relatively high radiation stability, different extraction properties and possibility to eliminate aqueous phase acidification (Cocalia et al, 2006a; Visser et al, 2000; Dai et al, 1999; Luo et al, 2006; Chen, 2007; Chun et al, 2001; Visser & Rogers, 2003). -

Hexaalkylguanidinium Salts As Ionic Liquids – New Applications In
Institut für Organische Chemie I Hexaalkylguanidinium Salts as Ionic Liquids – New Applications in Titanium and Aluminium Alcoholates Assisted Synthesis and as Electrolytes for Electrodeposition of Metals Dissertation zur Erlangung des Grades des Doktors Dr. rer. nat. der Fakultät für Naturwissenschaften der Universität Ulm vorgelegt von Dipl.-Ing. Maria Arkhipova aus Leningrad (St. Petersburg) Ulm 2014 Die vorliegende Arbeit entstand in der Zeit von Januar 2010 bis Januar 2014 in dem Institut für Organische Chemie I der Universität Ulm. Amtierender Dekan: Prof. Dr. Joachim Ankerhold 1. Gutachter: Prof. Dr. Gerhard Maas 2. Gutachter: Prof. Dr. Willi Kantlehner Tag der Promotion: 17.03.2014 Моим родителям List of abbreviations APIs Active Pharmaceutical Ingredients AES Auger Electron Spectroscopy BASIL Biphasic Acid Scavenging Utilising Ionic Liquids BINOL 1,1'-Bi-2-naphthol BMIm 1-Butyl-3-methylimidazolium BMPyr N-Butyl-N-methylpyrrolidinium Bn Benzyl Bu Butyl tBu tert-Butyl CHN Elemental analysis CI Chemical Ionisation CV Cyclic Voltammetry d day(s) DLS Dynamic Light Scattering DMC Dimethyl carbonate DMF Dimethylformamide DSC Differential Scanning Calorimetry DSSC Dye-Sensitised Solar Cell EC Ethylene carbonate EDX Energy-Dispersive X-ray analysis EIS Electrochemical Impedance Spectroscopy EMIm 1-Ethyl-3-methylimidazolium Et Ethyl FAP Tris(perfluoroalkyl)trifluorophosphate FSI Bis(fluorosulfonyl)imide GC Glassy Carbon Gu Guanidinium h hour(s) Hex Hexyl cHex Cyclohexyl HMBC Heteronuclear Multiple Bond Correlation HMMIm 1-Hexyl-2,3-dimethylimidazolium -

MDA) Analogs and Homologs
Terry A. Dal Cason, 1 M.Sc. An Evaluation of the Potential for Clandestine Manufacture of 3,4-Methylenedioxyamphetamine (MDA) Analogs and Homologs REFERENCE: Dal Cason, T. A., "An Evaluation of the Potential for Clandestine Manu- facture of 3,4-Methylenedioxyamphetamine (MDA) Analogs and Homologs," Journal of Forensic Sciences, JFSCA, Vol. 35, No.3, May 1990, pp. 675-697. ABSTRACT: Encountering a novel controlled-substance analog (designer drug) has become a distinct possibility for all forensic drug laboratories. 3,4-Methylenedioxyamphetamine (MDA) in particular is a receptive parent compound for the molecular modifications which produce such homo logs and analogs. The identification of these compounds, however, can prove to be an arduous task. It would be desirable to direct the focus of the identification to those compounds which are the more likely candidates for clandestine-laboratory synthesis. The process of narrowing the range of theoretical possibilities to logical choices may be enhanced by using a suitable predictive scheme. Such a predictive scheme for MDA analogs is presented based on putative Central Nervous System activity, existence or formulation of a reasonable synthesis method, and availability of the required precursors. KEYWORDS: toxicology, 3,4-methylenedioxyamphetamine, drug identification To circumvent statutes enacted to control the use of various dangerous drugs (controlled substances), clandestine laboratory operators will sometimes make minor alterations in the molecular structure of a parent compound. These structural changes are reflected in the chemical nomenclature of the new analog or homolog, and, in the past/ had effectively removed it from the purview of the law. Such modifications, at least in the case of 3,4- methylenedioxyamphetamine (MDA), do not appear to be haphazard. -

Synthesis and Applications of New Ionic Liquids
University of Pisa Department of Chemistry and Industrial Chemistry CHIM/06 Synthesis and Applications of New Ionic Liquids Tutor Prof. Cinzia Chiappe Candidate Angelo, Fabrizio Sanzone PhD in Chemical Sciences “Galileo Galilei” XXIV Cycle Abstract Ionic liquids (ILs) are promising new media constituted exclusively by ions which can be helpful in a variety of applications. The organic and ionic nature gives to these systems unique properties; a high solvent power towards organic, inorganic and polymeric compounds (including biopolymers) and a very low volatility (if any). This latter property determines the absence of solvent vapour in atmosphere thus avoiding explosive risk linked to that and the toxic effects for workers normally associated to the use of volatile organic solvents (VOC). Nevertheless, they have high polarity values, many have high conductivity values and low viscosity values as well. These properties are normally associated to an high thermal and electrochemical stability. It is to note that all the physico-chemical and biological properties of ionic liquids depend on the anion cation combination: the choice of the best combination of cation and anion is important to meet the requirements of any application. Due to their low volatility, high solvent power, recyclability ionic liquids are generally considered promising alternative solvents with a low environmental impact (green solvents). However, nowadays to increase the sustainability of many important chemical processes it is necessary not only to use eco-friendly reagents and solvents but it is important also reduce wastes (e.g. through the use of catalyst and solvent recovery) and to take into account these principles also when solvents or reagents are produced. -

Mechanistic Studies of Π-Activation Catalysis by Cationic Gold(I)
Mechanistic Studies of -Activation Catalysis by Cationic Gold(I) and Brønsted-acid by Rachel Elizabeth McKinney Brooner Department of Chemistry Duke University Date:_______________________ Approved: ___________________________ Ross A. Widenhoefer, Supervisor ___________________________ Steven W. Baldwin ___________________________ Katherine J. Franz ___________________________ Qiu Wang Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry in the Graduate School of Duke University 2013 ABSTRACT Mechanistic Studies of -Activation Catalysis by Cationic Gold(I) and Brønsted-acid by Rachel Elizabeth McKinney Brooner Department of Chemistry Duke University Date:_______________________ Approved: ___________________________ Ross A. Widenhoefer, Supervisor ___________________________ Steven W. Baldwin ___________________________ Katherine J. Franz ___________________________ Qiu Wang An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry in the Graduate School of Duke University 2013 i v Copyright by Rachel Brooner 2013 Abstract Soluble gold(I) complexes are highly efficient catalysts for the functionalization of C–C multiple bonds through the addition of carbon- or heteroatom-nucleophiles across -bonds or cycloisomerizations of enynes and related -systems. Mechanisms involving outer-sphere attack of a nucleophile on the electrophilic -ligand of a cationic gold -complex are typically invoked for gold(I)-catalyzed hydrofunctionalization and cycloisomerization processes, but direct experimental evidence for this mechanism is limited. As an extension of the pioneering research in the Widenhoefer lab on the synthesis and characterization of gold(I) -complexes, a diverse family of 15 gold(I) - complexes in three distinct series are reported herein. First, the synthesis, characterization, and solution behavior of a series of seven gold(I) -diene complexes is reported. -

Catalytic Radical-Polar Crossover Ritter Reaction Eric E
Catalytic Radical-Polar Crossover Ritter Reaction Eric E. Touney‡, Riley E. Cooper‡, Sarah. E. Bredenkamp, David T. George†, and Sergey V. Pronin* Department of Chemistry, University of California, Irvine, California 92697-2025, United States Supporting Information Placeholder Co(II) complex, ABSTRACT: A catalytic radical-polar crossover Ritter R3 R3 R3 silane, oxidant MeCN, H2O R1 R1 AcHN reaction is described. The transformation proceeds under R4 R4 R4 via R3 nuclephilic attack 1 2 acid-free conditions and tolerates a variety of functional R2 R2 & hydrolysis R R R1 R4 groups. The catalyst design overcomes limitations in the 32 examples substitution pattern of starting materials and enables hy- R2 droamidation of a diverse range of alkenes. Formation of Scheme 1. Catalytic radical-polar crossover Ritter reaction. hydrogen contributes to the background consumption of reductant and oxidant and competes with the desired path- polar crossover reactions of alkenes we observed that ap- way, pointing to a mechanistic link between hydrogen plication of acetonitrile as solvent occasionally resulted atom transfer-initiated organic reactions and hydrogen in formation of small amounts of side-products arising evolution catalysis. from apparent Markovnikov addition of acetamide to the carbon-carbon double bond.5,6 The electrophilic interme- diates in this formal Ritter reaction were proposed to arise from direct oxidation of alkyl radicals following the HAT Hydroamidation of alkenes with nitriles in the presence and diffusion from the solvent cage.7 Alternative path- of a strong Brønsted acid, discovered by Ritter, allows for ways involve radical pair collapse to generate the corre- a convenient access to tert-alkylamines and their deriva- 1 sponding alkylcobalt(III) complexes, which undergo oxi- tives from simple precursors. -

Digging Into Gold(I) Catalysis: Silver and Counterion Effects and Total Synthesis of Nardoaristolone B
DIGGING INTO GOLD(I) CATALYSIS: SILVER AND COUNTERION EFFECTS AND TOTAL SYNTHESIS OF NARDOARISTOLONE B. Anna Homs i Riba Dipòsit Legal: T 1605-2015 ADVERTIMENT. L'accés als continguts d'aquesta tesi doctoral i la seva utilització ha de respectar els drets de la persona autora. Pot ser utilitzada per a consulta o estudi personal, així com en activitats o materials d'investigació i docència en els termes establerts a l'art. 32 del Text Refós de la Llei de Propietat Intel·lectual (RDL 1/1996). Per altres utilitzacions es requereix l'autorització prèvia i expressa de la persona autora. En qualsevol cas, en la utilització dels seus continguts caldrà indicar de forma clara el nom i cognoms de la persona autora i el títol de la tesi doctoral. No s'autoritza la seva reproducció o altres formes d'explotació efectuades amb finalitats de lucre ni la seva comunicació pública des d'un lloc aliè al servei TDX. Tampoc s'autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant als continguts de la tesi com als seus resums i índexs. ADVERTENCIA. El acceso a los contenidos de esta tesis doctoral y su utilización debe respetar los derechos de la persona autora. Puede ser utilizada para consulta o estudio personal, así como en actividades o materiales de investigación y docencia en los términos establecidos en el art. 32 del Texto Refundido de la Ley de Propiedad Intelectual (RDL 1/1996). Para otros usos se requiere la autorización previa y expresa de la persona autora. -

Kinetics and Mechanism of Oxidation of Methyl Glycol and Ethyl Glycol by N-Bromosuccinimide in Alkaline Medium Catalysed by Os(VIII)
Oxidation Communications 36, No 3, 565–572 (2013) Oxidation in the presence of halogen-containing compounds KINETICS AND MECHANISM OF OXIDATION OF METHYL GLYCOL AND ETHYL GLYCOL BY N-BROMOSUCCINIMIDE IN ALKALINE MEDIUM CATALYSED BY Os(VIII) R. A. SINGHa*, KAMINI SINGHa, ABHISHEK KUMARa, S. K. SINGHb aChemical Kinetics Research Laboratory, Department of Chemistry, T. D. P. G. College, 222 002 Jaunpur (U.P.), India E-mail: [email protected]; [email protected] bDepartment of Chemistry, Sri J.N.P.G. College, Lucknow (U.P.), India ABSTRACT The kinetics of Os(VIII)-catalysed oxidation of methyl and ethyl glycol by N-bro mo- succinimide has been investigated in alkaline medium. The reaction shows first order kinetic with respect to methyl and ethyl glycols at their low concentration tended to zero order at their higher concentration. N-bromosuccinimide and osmium tetroxide also show first order kinetics. The dielectric constant of rate constant has positive effect. Negligible effect of variation of ionic strength of the medium on rate constant was observed. Increase in temperature markedly increase the rate constant of oxidation of methyl and ethyl glycols by N-bromosuccinimide under experimental condition. A plausible rate law can be proposed as – d[NBS] kdK1K2[NBS] [S] [Os(VIII)T][OH ] – = – – , dt 1 + K1[OH ] + K1K2 [OH ] [S] where S = glycol, viz. methyl and ethyl glycol. Keywords: oxidation, methyl glycol (MG), ethyl glycol (EG), osmium tetroxide, N-bromosuccinimide [NBS], kinetic and mechanistic approach. AIMS AND BACKGROUND Kinetic investigations involving aliphatic, cyclic, aromatic ketones and various oxidis- ing agents have been reported by many workers1,2. -
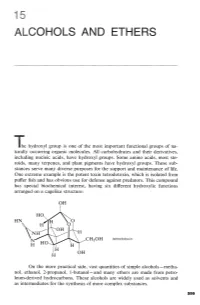
Alcohols and Ethers
ALCOHOLS AND ETHERS The hydroxyl group is one of the most important functional groups of na- turally occurring organic molecules. All carbohydrates and their derivatives, including nucleic acids, have hydroxyl groups. Some amino acids, most ste- roids, many terpenes, and plant pigments have hydroxyl groups. These sub- stances serve many diverse purposes for the support and maintenance of life. One extreme example is the potent toxin tetrodotoxin, which is isolated from puffer fish and has obvious use for defense against predators. This compound has special biochemical interest, having six different hydroxylic functions arranged on a cagelike structure: tetrodotoxin On the more practical side, vast quantities of simple alcohols - metha- nol, ethanol, 2-propanol, 1-butan01 - and many ethers are made from petro- leum-derived hydrocarbons. These alcohols are widely used as solvents and as intermediates for the synthesis of more complex substances. 15 Alcohols and Ethers The reactions involving the hydrogens of alcoholic OH groups are expected to be similar to those of water, HOH, the simplest hydroxylic com- pound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the 8-H bond but also with processes that result in cleavage of the C-0 bond, or changes in the organic group R. The simple ethers, ROR, do not have 0-H bonds, and most of their reactions are limited to the substituent groups. The chemistry of ethers, there- fore, is less varied than that of alcohols. This fact is turned to advantage in the widespread use of ethers as solvents for a variety of organic reactions, as we already have seen for Grignard reagents (Section 14- 10).