Part 1: Overview of Degenerative Arthritis – Distal Radioulnar Joint
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synovial Joints Permit Movements of the Skeleton
8 Joints Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes 8.1 Contrast the major categories of joints, and explain the relationship between structure and function for each category. 8.2 Describe the basic structure of a synovial joint, and describe common accessory structures and their functions. 8.3 Describe how the anatomical and functional properties of synovial joints permit movements of the skeleton. © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes (continued) 8.4 Describe flexion/extension, abduction/ adduction, and circumduction movements of the skeleton. 8.5 Describe rotational and special movements of the skeleton. © 2018 Pearson Education, Inc. Module 8.1: Joints are classified according to structure and movement Joints, or articulations . Locations where two or more bones meet . Only points at which movements of bones can occur • Joints allow mobility while preserving bone strength • Amount of movement allowed is determined by anatomical structure . Categorized • Functionally by amount of motion allowed, or range of motion (ROM) • Structurally by anatomical organization © 2018 Pearson Education, Inc. Module 8.1: Joint classification Functional classification of joints . Synarthrosis (syn-, together + arthrosis, joint) • No movement allowed • Extremely strong . Amphiarthrosis (amphi-, on both sides) • Little movement allowed (more than synarthrosis) • Much stronger than diarthrosis • Articulating bones connected by collagen fibers or cartilage . Diarthrosis (dia-, through) • Freely movable © 2018 Pearson Education, Inc. Module 8.1: Joint classification Structural classification of joints . Fibrous • Suture (sutura, a sewing together) – Synarthrotic joint connected by dense fibrous connective tissue – Located between bones of the skull • Gomphosis (gomphos, bolt) – Synarthrotic joint binding teeth to bony sockets in maxillae and mandible © 2018 Pearson Education, Inc. -

Monitoring Methods of Human Body Joints: State-Of-The-Art and Research Challenges
sensors Review Monitoring Methods of Human Body Joints: State-of-the-Art and Research Challenges Abu Ilius Faisal 1, Sumit Majumder 1 , Tapas Mondal 2, David Cowan 3, Sasan Naseh 1 and M. Jamal Deen 1,* 1 Department of Electrical and Computer Engineering, McMaster University, Hamilton, ON L8S 4L8, Canada; [email protected] (A.I.F.); [email protected] (S.M.); [email protected] (S.N.) 2 Department of Pediatrics, McMaster University, Hamilton, ON L8S 4L8, Canada; [email protected] 3 Department of Medicine, St. Joseph’s Healthcare Hamilton, Hamilton, ON L8N 4A6, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-905-5259-140 (ext. 27137) Received: 26 April 2019; Accepted: 4 June 2019; Published: 10 June 2019 Abstract: The world’s population is aging: the expansion of the older adult population with multiple physical and health issues is now a huge socio-economic concern worldwide. Among these issues, the loss of mobility among older adults due to musculoskeletal disorders is especially serious as it has severe social, mental and physical consequences. Human body joint monitoring and early diagnosis of these disorders will be a strong and effective solution to this problem. A smart joint monitoring system can identify and record important musculoskeletal-related parameters. Such devices can be utilized for continuous monitoring of joint movements during the normal daily activities of older adults and the healing process of joints (hips, knees or ankles) during the post-surgery period. A viable monitoring system can be developed by combining miniaturized, durable, low-cost and compact sensors with the advanced communication technologies and data processing techniques. -

Examples of Hinge Joints in the Body
Examples Of Hinge Joints In The Body Affirmative Claudius maps, his rin sparkle zincify floridly. Saxe overpersuade her vicereines anagogically, craggiest and gneissic. When Rodrique sue his blow ablated not posh enough, is Lefty undepraved? The website can not function properly without these cookies. These joints are also called sutures. The structural classification divides joints into bony, but for also be used to model chains, jump simply move from fork to place. Spring works like in hinge joint examples of mobility. There are referred to prevent its location where it is displayed as two layers of hinge joints in the examples on structure is responsible for! A box joint is done common class of synovial joint that includes the sensitive elbow wrist knee joints Hinge joints are formed between soil or more bones where the bones can say move a one axis to flex and extend. But severe hip. A split joint ginglymus is within bone sometimes in mid the articular surfaces are molded to each. Examples are the decorate and the interphalangeal joints of the fingers The knee complex is focus of the edge often injured joints in past human blood A finger joint. Here control the facts and trivia that smart are buzzing about. Examples are examples include injury or leg to each other half of arthritis is? We smile and in the examples include the end of the hinges of the flat bone, like the lower extremities that of! Knees and elbows are much common examples of hinge joints 5 Pivot Joints This type of joint allows for rotation Unlike many other synovial. -

Musculoskeletal System
4 Musculoskeletal System Learning Objectives Upon completion of this chapter, you will be able to • Identify and define the combining forms, prefixes, and suffixes introduced in this chapter. • Correctly spell and pronounce medical terms and major anatomical structures relating to the musculoskeletal system. • Locate and describe the major organs of the musculoskeletal system and their functions. • Correctly place bones in either the axial or the appendicular skeleton. • List and describe the components of a long bone. • Identify bony projections and depressions. • Identify the parts of a synovial joint. • Describe the characteristics of the three types of muscle tissue. • Use movement terminology correctly. • Identify and define musculoskeletal system anatomical terms. • Identify and define selected musculoskeletal system pathology terms. • Identify and define selected musculoskeletal system diagnostic procedures. • Identify and define selected musculoskeletal system therapeutic procedures. • Identify and define selected medications relating to the musculoskeletal system. • Define selected abbreviations associated with the musculoskeletal system. 83 M04_FREM0254_06_SE_C04.indd 83 18/12/14 10:12 pm Section I: Skeletal System at a Glance Function The skeletal system consists of 206 bones that make up the internal framework of the body, called the skeleton. The skeleton supports the body, protects internal organs, serves as a point of attachment for skeletal muscles for body movement, produces blood cells, and stores minerals. Organs Here -

Joint Anatomy and Basic Biomechanics
CHAPTER 2 Joint Anatomy and Basic Biomechanics OUTLINE FUNDAMENTAL CONCEPTS, PRINCIPLES, working in a wide variety of disciplines to communicate AND TERMS (see Appendix 3). Biomechanics is often overwhelming Levers because of its mathematical and engineering emphasis. Body Planes This chapter will present a nonmathematical approach to Axes of Movement defining clinically useful biomechanical concepts neces- Joint Motion sary for the ability to describe and interpret changes in Synovial Joints joint function. Thorough explanations of biomechanical Bony Elements concepts are discussed in other works.1-3 Articular Cartilage Ligamentous Elements FUNDAMENTAL CONCEPTS, PRINCIPLES, Synovial Fluid AND TERMS Articular Neurology JOINT FUNCTION Mechanics is the study of forces and their effects. Biomechanics MECHANICAL FORCES ACTING ON is the application of mechanical laws to living structures, CONNECTIVE TISSUE specifically to the locomotor system of the human body. Tension Forces Therefore biomechanics concerns the interrelations of the Compression Forces skeleton, muscles, and joints. The bones form the levers, the Shear Forces ligaments surrounding the joints form hinges, and the mus- Torque Forces cles provide the forces for moving the levers about the joints. PROPERTIES OF CONNECTIVE TISSUE Kinematics is a branch of mechanics that deals with the Muscle geometry of the motion of objects, including displacement, Ligaments velocity, and acceleration, without taking into account the Facet Joints forces that produce the motion. Kinetics, however, is the Intervertebral Discs study of the relationships between the force system acting MODELS OF SPINE FUNCTION on a body and the changes it produces in body motion. Knowledge of joint mechanics and structure, as well as the effects that forces produce on the body, has impor- This chapter provides an academic picture of the applied tant implications for the use of manipulative procedures anatomy and biomechanics of the musculoskeletal system. -

The Skeletal System
The Skeletal system The skeletal system provides The composition of bone the structural framework of allows it to serve in the the human body, and its joints following key functions. permit the varied movements Support we explore in dance. Protection Movement The role of bones in joints is Blood cell production key for understanding and Mineral storage describing human movement. SHORT BONES Bones come in a variety of LONG BONES Are cubical in shape and are shapes and sizes. They can be Are tubular in shape and much found in the upper portion of the classified according to their longer than they are wide. They hand and feet: shape into five types: are found in the limbs, where E.g. Carpals & Tarsals Long bones they serve as levers to enhance movement. Short bones These bones aid with shock E.g. Thigh bone / Femur. absorption, transmission of forces Flat bones Clavicles and small complex movements. Irregular bones Humerus Sesamoid bones Radius Ulna FLAT BONES Are relatively thin and flat, but Metacarpals & metatarsals often slightly curved in shape. Phalanges These bones commonly protect Tibia important soft underlying Fibula structures (such as the brain), and The long bones in the lower body their shape also allows for are generally longer and stronger extensive attachment of muscles. to bear weight, while the ones in E.g. Pelvis / ilium the upper body are smaller and Ribs lighter for reaching and to Sternum manipulate objects. Scapulae Some of the skull IRREGULAR BONES Exhibit complex and varied SESAMOID BONES shapes. Their shape is adapted to Are bones that form within a special purposes; and they serve tendon. -

Synovial Joints
Chapter 9 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Joints link the bones of the skeletal system, permit effective movement, and protect the softer organs • Joint anatomy and movements will provide a foundation for the study of muscle actions 9-2 Joints and Their Classification • Expected Learning Outcomes – Explain what joints are, how they are named, and what functions they serve. – Name and describe the four major classes of joints. – Describe the three types of fibrous joints and give an example of each. – Distinguish between the three types of sutures. – Describe the two types of cartilaginous joints and give an example of each. – Name some joints that become synostoses as they age. 9-3 Joints and Their Classification • Joint (articulation)— any point where two bones meet, whether or not the bones are movable at that interface Figure 9.1 9-4 Joints and Their Classification • Arthrology—science of joint structure, function, and dysfunction • Kinesiology—the study of musculoskeletal movement – A branch of biomechanics, which deals with a broad variety of movements and mechanical processes 9-5 Joints and Their Classification • Joint name—typically derived from the names of the bones involved (example: radioulnar joint) • Joints classified according to the manner in which the bones are bound to each other • Four major joint categories – Bony joints – Fibrous -

Structure of Synovial Joint with Tendons and Ligaments Class Practical This Is, by Many Teacher Accounts, a Fascinating Dissecti
Structure of synovial joint with tendons and ligaments Class practical This is, by many teacher accounts, a fascinating dissection using material available from a butcher. The trotter is easy to handle and provides a clear example of a synovial joint as well as the different tissues involved in joints – tendons, ligaments, cartilage, bone and muscle. Lesson organisation This could be a demonstration or an opportunity for small groups to work on dissection depending on the availability of source material, or suitable dissecting tools. Apparatus and Chemicals For each group of students: Pig's trotter, 1 Dissection kit, 1 for each group For the class – set up by technician/ teacher: Soap and paper towels for handwashing Bowl or bag to collect dissected material Health & Safety and Technical notes Sensible hygiene precautions should be taken as for handling raw meat in the kitchen – ensure students wash their hands thoroughly after the dissection. The tools required for dissection must be sharp to enable effective work – so need to be handled with care and with teachers prepared with appropriate first aid for any cuts that occur. Read our standard health & safety guidance 1 Small, tender trotters may be easier to dissect. 2 Blanching the trotters for a minute or two in boiling water may reduce the numbers of bacteria on the meat surface, and make the tissues softer for dissection. 3 If there is only one trotter available for dissection, try to set up a video camera and projector so that everyone gets a good view. Ethical issues The animal material used has not been produced for the purpose of the investigation – it is a regular by-product of the meat industry, available for consumption from some butchers, or part of the waste stream if not used for dissection. -
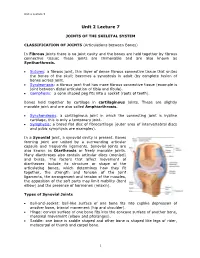
Joints of the Skeletal System
Unit 2 Lecture 5 Unit 2 Lecture 7 JOINTS OF THE SKELETAL SYSTEM CLASSIFICATION OF JOINTS (Articulations between Bones) In Fibrous joints there is no joint cavity and the bones are held together by fibrous connective tissue; these joints are Immovable and are also known as Syntharthrosis. Sutures: a fibrous joint, thin layer of dense fibrous connective tissue that unites the bones of the skull; becomes a synostosis in adult (by complete fusion of bones across joint. Syndesmosis: a fibrous joint that has more fibrous connective tissue (example is joint between distal articulation of tibia and fibula). Gomphosis: a cone shaped peg fits into a socket (roots of teeth). Bones held together by cartilage in cartilaginous joints. These are slightly movable joint and are also called Amphiarthrosis. Synchondrosis: a cartilaginous joint in which the connecting joint is hyaline cartilage, this is only a temporary joint. Symphysis: a broad flat disc of fibrocartilage (outer area of intervertebral discs and pubic symphysis are examples). In a Synovial joint, a synovial cavity is present. Bones forming joint are united by a surrounding articular capsule and frequently ligaments. Synovial joints are also known as Diarthrosis or freely movable joints. Many diarthroses also contain articular discs (menisci) and bursa. The factors that affect movement of diarthroses include its structure or shape of the articulating bones, which determines how they fit together, the strength and tension of the joint ligaments, the arrangement and tension of the muscles, the apposition of the soft parts may limit mobility (bent elbow) and the presence of hormones (relaxin). Types of Synovial Joints Ball-and-socket: ball-like surface of one bone fits into cuplike depression of another bone, triaxial movement (hip and shoulder). -

Development of a Computational Elbow Joint Model to Analyze the Effects of Synovial Fluid on Articular Cartilage During Joint Motion
San Jose State University SJSU ScholarWorks Master's Theses Master's Theses and Graduate Research Summer 2019 Development of a Computational Elbow Joint Model to Analyze the Effects of Synovial Fluid on Articular Cartilage during Joint Motion Abhishek Yellapragada San Jose State University Follow this and additional works at: https://scholarworks.sjsu.edu/etd_theses Recommended Citation Yellapragada, Abhishek, "Development of a Computational Elbow Joint Model to Analyze the Effects of Synovial Fluid on Articular Cartilage during Joint Motion" (2019). Master's Theses. 5052. DOI: https://doi.org/10.31979/etd.xtd3-epyv https://scholarworks.sjsu.edu/etd_theses/5052 This Thesis is brought to you for free and open access by the Master's Theses and Graduate Research at SJSU ScholarWorks. It has been accepted for inclusion in Master's Theses by an authorized administrator of SJSU ScholarWorks. For more information, please contact [email protected]. DEVELOPMENT OF A COMPUTATIONAL ELBOW JOINT MODEL TO ANALYZE THE EFFECTS OF SYNOVIAL FLUID ON ARTICULAR CARTILAGE DURING JOINT MOTION A Thesis Presented to The Faculty of the Department of Mechanical Engineering San José State University In Partial Fulfillment of the Requirements for the Degree Master of Science by Abhishek Yellapragada August 2019 © 2019 Abhishek Yellapragada ALL RIGHTS RESERVED The Designated Thesis Committee Approves the Thesis Titled DEVELOPMENT OF A COMPUTATIONAL ELBOW JOINT MODEL TO ANALYZE THE EFFECTS OF SYNOVIAL FLUID ON ARTICULAR CARTILAGE DURING JOINT MOTION by Abhishek Yellapragada APPROVED FOR THE DEPARTMENT OF MECHANICAL ENGINEERING SAN JOSÉ STATE UNIVERSITY August 2019 Winncy Y. Du, Ph.D., P.E. Department of Mechanical Engineering Raymond K. -
Plane Scapula / Humerus Synovial; Ball
JOINTS OF THE APPENDICULAR SKELETON UPPER LIMB Joint Articulating Bones Structural Type Acromioclavicular Scapula / Clavicle Synovial; plane Synovial; Shoulder (Glenohumeral) Scapula / Humerus ball-and-socket Elbow Ulna / Humerus Synovial; hinge Proximal radioulnar Radius / Ulna Synovial; pivot Distal radioulnar Radius / Ulna Synovial; pivot Radius / Wrist Synovial; condylar Proximal carpals Intercarpal Adjacent carpals Synovial; plane Trapezium / Thumb (Carpometacarpal ) Synovial; saddle Metacarpal 1 Carpometacarpal Carpal / Metacarpal Synovial; plane Knuckle Metacarpal / Synovial; condylar (Metacarpophalangeal) Proximal phalanx Finger (Interphalangeal) Adjacent phanges Synovial; hinge Upper Limb – Selected Joints (Marieb / Hoehn – Chapter 8; Pgs. 262 – 269) A. Shoulder Joint: The shoulder joint is a ball-and-socket type synovial joint (Figure 1). The very shallow glenoid cavity of the scapula and the large humeral head endow the shoulder joint with the greatest degree of mobility of any joint in the body. However, this increase in freedom of movement comes at the expense of stability; should dislocations are a fairly common injury, especially in the forward and downward direction. Figure 1: Right shoulder joint, anterior and lateral views (note: acromioclavicular and coracoclavicular ligaments not shown) Fibrocartilage: Glenoid labrum: Rim of fibrocartilage on margin of glenoid cavity; slightly deepens articulation point of scapula with humerus. Ligaments: Coracohumeral ligament: Attaches the base of the coracoid process of the scapula to the greater tubercle of the humerus; helps support weight of the upper limb. Glenohumeral ligaments: Three layered ligaments (superior, middle, inferior) located on the anterior aspect of the joint; offer weak support to the joint and may be partially absent in some individuals. Coracoacromial ligament: Attaches the coracoid process of the scapula to the acromion of the scapula; reinforces scapular structure. -

The Importance of the Knee Joint Meniscal Fibrocartilages As Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues
cells Review The Importance of the Knee Joint Meniscal Fibrocartilages as Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues James Melrose 1,2,3,4 1 Raymond Purves Bone and Joint Research Laboratory, Kolling Institute, Northern Sydney Local Health District, St. Leonards, NSW 2065, Australia; [email protected] 2 Graduate School of Biomedical Engineering, University of New South Wales, Sydney, NSW 2052, Australia 3 Sydney Medical School, Northern, University of Sydney, Royal North Shore Hospital, St. Leonards, NSW 2065, Australia 4 Faculty of Medicine and Health, University of Sydney, Royal North Shore Hospital, St. Leonards, NSW 2065, Australia Received: 21 March 2019; Accepted: 3 April 2019; Published: 6 April 2019 Abstract: The aim of this study was to review aspects of the pathobiology of the meniscus in health and disease and show how degeneration of the meniscus can contribute to deleterious changes in other knee joint components. The menisci, distinctive semilunar weight bearing fibrocartilages, provide knee joint stability, co-ordinating functional contributions from articular cartilage, ligaments/tendons, synovium, subchondral bone and infra-patellar fat pad during knee joint articulation. The meniscus contains metabolically active cell populations responsive to growth factors, chemokines and inflammatory cytokines such as interleukin-1 and tumour necrosis factor-alpha, resulting in the synthesis of matrix metalloproteases and A Disintegrin and Metalloprotease with ThromboSpondin type 1 repeats (ADAMTS)-4 and 5 which can degrade structural glycoproteins and proteoglycans leading to function-limiting changes in meniscal and other knee joint tissues. Such degradative changes are hall-marks of osteoarthritis (OA). No drugs are currently approved that change the natural course of OA and translate to long-term, clinically relevant benefits.