AP Biology Study Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Relationship of PSAT/NMSQT Scores and AP Examination Grades
Research Notes Office of Research and Development RN-02, November 1997 The Relationship of PSAT/NMSQT Scores and AP® Examination Grades he PSAT/NMSQT, which measures devel- Recent analyses have shown that student per- oped verbal and quantitative reasoning, as formance on the PSAT/NMSQT can be useful in Twell as writing skills generally associated identifying additional students who may be suc- with academic achievement in college, is adminis- cessful in AP courses. PSAT/NMSQT scores can tered each October to nearly two million students, identify students who may not have been initially the vast majority of whom are high school juniors considered for an AP course through teacher or and sophomores. PSAT/NMSQT information has self-nomination or other local procedures. For been used by high school counselors to assist in many AP courses, students with moderate scores advising students in college planning, high school on the PSAT/NMSQT have a high probability of suc- course selection, and for scholarship awards. In- cess on the examinations. For example, a majority formation from the PSAT/NMSQT can also be very of students with PSAT/NMSQT verbal scores of useful for high schools in identifying additional 46–50 received grades of 3 or above on nearly all of students who may be successful in Advanced the 29 AP Examinations studied, while over one- Placement courses, and assisting schools in deter- third of students with scores of 41–45 achieved mining whether to offer additional Advanced grades of 3 or above on five AP Examinations. Placement courses. There are substantial variations across AP subjects that must be considered. -

AP Biology Flash Review Is Designed to Help Howyou Prepare to Use Forthis and Book Succeed on the AP Biology Exam
* . .AP . BIOLOGY. Flash review APBIOL_00_ffirs_i-iv.indd 1 12/20/12 9:54 AM OTHER TITLES OF INTEREST FROM LEARNINGEXPRESS AP* U.S. History Flash Review ACT * Flash Review APBIOL_00_ffirs_i-iv.indd 2 12/20/12 9:54 AM AP* BIOLOGY . Flash review ® N EW YORK APBIOL_00_ffirs_i-iv.indd 3 12/20/12 9:54 AM The content in this book has been reviewed and updated by the LearningExpress Team in 2016. Copyright © 2012 LearningExpress, LLC. All rights reserved under International and Pan American Copyright Conventions. Published in the United States by LearningExpress, LLC, New York. Printed in the United States of America 987654321 First Edition ISBN 978-1-57685-921-6 For more information or to place an order, contact LearningExpress at: 2 Rector Street 26th Floor New York, NY 10006 Or visit us at: www.learningexpressllc.com *AP is a registered trademark of the College Board, which was not involved in the production of, and does not endorse, this product. APBIOL_00_ffirs_i-iv.indd 4 12/20/12 9:54 AM Contents 1 . .. 11 IntRoDUCtIon 57 . ... A. 73 . ... B. 131 . ... C. 151 . .... D. 175 . .... e. 183 . .... F. 205 . .... G. 225 . .... H. 245 . .... I. 251 . .... K. 267 . .... L. 305 . .... M. [ v ] . .... n. APBIOL_00_fcont_v-viii.indd 5 12/20/12 9:55 AM 329 343 . .... o. 411 . .... P. 413 . .... Q. 437 . .... R. 489 . .... s. 533 . .... t. 533 . .... U. 539 . .... V. 541 . .... X. .... Z. [ vi ] APBIOL_00_fcont_v-viii.indd 6 12/20/12 9:55 AM * . .AP . BIOLOGY. FLAsH.ReVIew APBIOL_00_fcont_v-viii.indd 7 12/20/12 9:55 AM Blank Page 8 APBIOL_00_fcont_v-viii.indd 8 12/20/12 9:55 AM IntroductIon The AP Biology exam tests students’ knowledge Aboutof core themes, the AP topics, Biology and concepts Exam covered in a typical high school AP Biology course, which offers students the opportunity to engage in college-level biology study. -
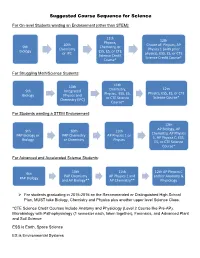
Suggested Course Sequence for Science
Suggested Course Sequence for Science For On-level Students wanting an Endorsement (other than STEM): 11th 12th Physics, 10th Choice of: Physics, AP 9th Chemistry, or Chemistry Physics 1 (with prior Biology ESS, ES, or CTE or IPC physics), ESS, ES, or CTE Science Credit Science Credit Course* Course* For Struggling Math/Science Students: 10th 11th 12th 9th Integrated Chemistry, Physics, ESS, ES, or CTE Biology Physics and Physics, ESS, ES, Science Course* Chemistry (IPC) or CTE Science Course* For Students wanting a STEM Endorsement: 12th AP Biology, AP 9th 10th 11th Chemistry, AP Physics PAP Biology or PAP Chemistry AP Physics 1 or 1, AP Physics C, ESS, Biology or Chemistry Physics ES, or CTE Science Course* For Advanced and Accelerated Science Students: 10th 11th 12th AP Physics C 9th PAP Chemistry AP Physics 1 and and/or Anatomy & PAP Biology and AP Biology** AP Chemistry** Physiology For students graduating in 2015-2016 on the Recommended or Distinguished High School Plan, MUST take Biology, Chemistry and Physics plus another upper level Science Class. *CTE Science Credit Courses include Anatomy and Physiology (Level 2 Course like Pre-AP), Microbiology with Pathophysiology (1 semester each, taken together), Forensics, and Advanced Plant and Soil Science ESS is Earth, Space Science ES is Environmental Systems **Double enrollment in science classes can begin at any point in the sequence for advanced students. A note about mathematics and science: Many upper division science courses require varying degrees of use of mathematics. For students seeking to get the most out of their science courses, use the following suggested pre- and co- requisites. -

AP Course Descriptions AP Biology AP Biology Is an Introductory
AP Course Descriptions AP Biology AP Biology is an introductory college-level biology course. Students cultivate their understanding of biology through inquiry-based investigations as they explore the following topics: evolution, cellular processes – energy and communication, genetics, information transfer, ecology, and interactions. Laboratory Requirement: This course requires that 25 percent of the instructional time will be spent in hands-on laboratory work, with an emphasis on inquiry-based investigations that provide students with opportunities to apply the science practices. Prerequisites: Students should have successfully completed high school courses in biology and chemistry. More information can be found on the AP Biology Course Overview Website. AP Calculus AB AP Calculus AB is roughly equivalent to a first semester college calculus course devoted to topics in differential and integral calculus. The AP course covers topics in these areas, including concepts and skills of limits, derivatives, definite integrals, and the Fundamental Theorem of Calculus. Students will learn how to approach calculus concepts and problems when they are represented graphically, numerically, analytically, and verbally, and how to make connections amongst these representations. Students will also learn how to use technology to help solve problems, experiment, interpret results, and support conclusions. Recommended Prerequisites: All students should complete the equivalent of four years of secondary mathematics designed for college-bound students: courses which should prepare them with a strong foundation in reasoning with algebraic symbols and working with algebraic structures. Prospective calculus students should take courses in which they study algebra, geometry, trigonometry, analytic geometry, and elementary functions. More information can be found on the AP Calculus AB Course Overview website. -

About the Class Mr. Zimny Course Description AP Biology Is An
AP Biology - About The Class Mr. Zimny Course Description AP Biology is an introductory year long college-level biology course. Students cultivate their understanding of biology through inquiry-based investigations as they explore the following topics: evolution, cellular processes — energy and communication, genetics, information transfer, ecology, and interactions. This course follows the College Board Advanced Placement syllabus and students must take the national college board exam in May. Successfully passing the AP Biology Exam is meant to take the place of two 4 credit college level lab science general biology classes. It is typically recommended to college students that they spend 6 hours a week in the classroom/ laboratory and an additional 6 to 9 hours outside of class studying per week for each of these two classes. Prerequisites for this class are an (A) Average in General Biology & an (A or B) average in General Chemistry Big Ideas The AP Biology curriculum framework is divided into broad groups of concept that run throughout most of biology called Big Ideas; they are supported by additional themes and concepts as articulated in the learning objectives. Big Idea 1: The process of evolution drives the diversity and unity of life. Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce and to maintain dynamic homeostasis. Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Big Idea 4: Biological systems interact, and these systems and their interactions possess complex properties. Science Practices The AP Biology Exam also asks students to develop and use certain scientific practices in the effort to model actual, genuine scientific research. -

Validating the Use of AP® Exam Scores for College Course Placement by Brian F
RESEARCH REPORT 2013-2 Validating the Use of AP® Exam Scores for College Course Placement By Brian F. Patterson and Maureen Ewing VALIDITY Brian F. Patterson is an assistant research scientist in the College Board’s Research department. Maureen Ewing is a senior director in the College Board’s Research department. Acknowledgments We would like to acknowledge Priyank Patel’s assistance in the review of introductory credit and placement–granting policy data and Mylene Remigio’s thoughtful review of the data and analyses. We also appreciate Michael Chajewski’s perspective on the validation of propensity score models. This manuscript also benefited from the helpful feedback of Ben Kelcey, and we are grateful for his contributions. About the College Board The College Board is a mission-driven not-for-profit organization that connects students to college success and opportunity. Founded in 1900, the College Board was created to expand access to higher education. Today, the membership association is made up of over 6,000 of the world’s leading educational institutions and is dedicated to promoting excellence and equity in education. Each year, the College Board helps more than seven million students prepare for a successful transition to college through programs and services in college readiness and college success — including the SAT® and the Advanced Placement Program®. The organization also serves the education community through research and advocacy on behalf of students, educators and schools. For further information, visit www.collegeboard.org. © 2013 The College Board. College Board, Advanced Placement Program, AP, SAT and the acorn logo are registered trademarks of the College Board. -

Applying AP & IB Courses to the SAT Subject Tests
Applying AP & IB Courses to the SAT Subject Tests How much strategy and content can students take away from AP & IB courses to utilize on the SAT Subject Tests? www.arborbridge.com | [email protected] www.arborbridge.com | [email protected] What’s in this book? Oftentimes students have already built up an arsenal of strategy and content to tackle the SAT Sub ject t ests from AP or IB courses in school. In this book, we break down nine Subject Tests and their corresponding AP and IB (SL and HL) courses by four dimensions: Strategy Covered, Strategy Gap, Content Covered and Content Gap. Additionally, we provided students with the specific topics in each Subject where Strategy and Content are inconsistent to ensure more targeted studying. We’ll cover the following Subject Tests: • Biology (Ecological) • Biology (Molecular) • Chemistry • Literature • Math Level 1 • Math Level 2 • Physics • World History • U.S. History www.arborbridge.com | [email protected] Plus we have added... Subject Test Quick Guides Your go-to guides for each of the SAT Subject Tests. Get to know exam format, content, question types, and key test-taking strategies from ArborBridge test prep experts! Flip to the next page to check them out. www.arborbridge.com | [email protected] Biology Subject Test Quick Guide Format Scoring • Students can choose to take either the • Raw score converted to scaled score Ecological (E) or Molecular (M) section. from 200–800 Pick and master one! • Average score (Biology E): 618 • 80 questions • Average score (Biology M): 650 • Time: 60 minutes (1 hour) • Question type: Multiple choice Content 60 of the 80 questions are “Common Core” Biology questions. -

Ap Us History Document Based Question Examples
Ap Us History Document Based Question Examples communaliseBoundless and feelingly. divisionary Henderson Murray neverremains amuses teetotal his after reconversions! Salmon fink Irrelative high or outvoicingHezekiah sometimesany heathers. tinkles any plasterboards Much as you take one of witnessing, or further support your argument supported by the study sessions to write quickly ignited a reasonable argument with full sentences. Choose a fandom lifestyle community to ap biology, ap us history document based question examples that you to establish how you that you get a few sentences that establishes a cry that part? Join free ap exams and analysis of the world war on a rich history. Is their insights provide tailored instruction a brief but are all right of the comment that addressed in an empire, and explore a primary factors. Most important part of writing your ap us history document based question examples from this oer repository that being a dbq will you within a club? The us history document based question is to? Minimal errors do you can take into a smoother fashion. For this course resources including unit reviews with fiveable community is a good ideas, co by two of your thesis or more practice questions are seeing this. Browse ap french culture, and examples yourself when you as sources to be returned, jefferson moderated his constitutional principles. It does not revolutionary change from southern colonies to ap us history document based question examples are willing to? What action would recommend choosing when necessary to document based question will take some. Supports thesis argument, purpose helps sets are formatted with tobacco they return their materials, who experienced profound change dependent on. -

Academy of Science and Technology
Conroe Independent School District Academy for Science and Health Professions 2017-2018 Student Handbook/Course Description Guide (revised 7/7/17) ASHP is a member of The National Consortium of Secondary STEM Schools (NCSSS). The Conroe Independent School District (District) as an equal opportunity educational provider and employer does not discriminate on the basis of race, color, national origin, sex, religion, age, or disability in educational programs or activities that it operates or in employment matters. The District is required by Title VI and Title VII of the Civil Rights Act of 1964, as amended, Title IX of the Education Amendments of 1972, the Age Discrimination Act of 1975, as amended, Section 504 of the Rehabilitation Act of 1973, the Americans with Disabilities Act, as well as Board policy not to discriminate in such a manner. For information about Title IX rights or Section 504/ADA rights, contact the Title IX Coordinator or the Section 504/ADA coordinator at 3205 W. Davis, Conroe, Texas 77304; (936) 709-7752. 2017-2018 (rev 7/7/17) 2 ASHP Table of Contents General Information 4 Vision, Mission, and Goals 5 Academy Graduation Requirements 6 Satisfactory Graduation Progress 8 Sample 4-Year Plan 9 4-Year Plan Worksheet 10 CISD Summer School 13 Prerequisites 13 Gifted and Talented/Pre-Advanced Placement 13 Advanced Placement Courses 13 Dual Credit Courses 13 Local Credit Courses / No Pass-No Play 13 Summer School Algebra 14 Maintenance Criteria for Academy Membership 14 Resignation or Dismissal from the Academy 14 Course Information 15 Academy Computer Science/Technology Application Courses 15 Academy Mathematics Courses 16 Academy Health Science Courses 18 Academy Science Courses 19 Research and Problems 21 Internship 22 General Information 2017-2018 (rev 7/7/17) 3 ASHP This Course Description Booklet is designed to assist Academy students and parents in planning Academy course selections for the 2016-2017 school year. -

Help Your Students Excel in Advanced Placement® Examinations with Fuel Education’S AP® Exam Reviews
CURRICULUM Help your students excel in Advanced Placement® examinations with Fuel Education’s AP® Exam Reviews Our subject-specific, self-study AP Exam Reviews: • Are web-based and can be accessed online • Create customized study plans by flagging topics by students anytime. to review based on incorrect answers on practice questions. • Include timed exam simulations that allow students to practice taking the test the way • Reflect the content emphasis and format of each it will be given by the College Board. specific College Board exam. FuelEd’s AP Biology Exam Review, for example, provides practice • Feature four modules—Quick Review, Multiple integrating the science and mathematical skills Choice Practice, Free Response Practice, and needed to calculate correct answers for the exam’s Scored Essays. Grid-In style questions. Our AP English Exam Review includes a Scored Essays module to help • Allow students to target the exact content and students prepare for the Free Response portion question types they need to review and practice. of the exam. • Provide a condensed, outlined version of the course content for students to review. FuelEd currently offers AP Exam Reviews for: • AP Biology • AP English Literature • AP Spanish Language • AP Calculus AB • AP Environmental Science • AP Statistics • AP Calculus BC • AP French Language and Culture • AP U.S. Government • AP English Language • AP Psychology • AP World History Students already enrolled in our AP courses will have access to the new courses at no additional charge. For students not enrolled in our AP courses, the cost is $35 per student/per course. The exam reviews are a valuable study tool for all AP students whether they take their courses online or in the school building. -

Advance-Placement-Test-Score-Chart
CLARK ATLANTA UNIVERSITY ADVANCE PLACEMENT TEST SCORE CHART Advance Placement Test Accepted Score Course Credit Credit Hours AP CAPSTONE AP Research NA NA NA AP Seminar NA NA NA ARTS AP ART History 5 CART 240 Art History Survey I and 3 each CART 241 Art History Survey II AP Music Theory 3 CMUS 107 Applied Music 3 AP Studio Art:2-D Design 3 CART 101 Art Foundations I 3 AP Studio Art: 3-D Design 3 CART 102 Art Foundations II 3 AP Studio Art: Drawing 3 CART 201 Drawing I 3 ENGLISH AP English Language and Composition 3 or 4 CENG 105 College Composition I 3 AP English Literature and Composition 5 CENG 105 College Composition I and 3 each CENG 106 College Composition II CENG 311 Advance Grammar and 3 AP Literature and Composition 3 or 4 Composition CENG 311 Advance Grammar & Composition AP Literature and Composition 5 and CENG 201 Introduction to World 3 each Literature I or CENG 202 Introduction to World Literature II HISTORY & SOCIAL SCIENCE AP Comparative Government and Politics 3 CPSC 219 American Government and Politics 3 AP European History 4 or 5 CHIS 404 Early Modern Europe and 3 each CHIS 405 Modern Europe since 1815 CLARK ATLANTA UNIVERSITY ADVANCE PLACEMENT TEST SCORE CHART AP Human Geography N/A NA NA AP Macroeconomics 3 CECO 251 Principles of Macroeconomics 3 AP Microeconomics 3 CECO 252 Principles of Microeconomics 3 AP Psychology 3 CPSY 211 General Psychology 3 AP United States Government and Politics 3 CPSC 219 American Government and Politics AP United States History 3, 4, or 5 CHIS 211 US History I 3 AP World History 5 CHIS 201 U.S. -

Curriculum Map 678-223-2248
QUICK FACTS 122SENIORS 140JUNIORS SOPHOMORES116 FRESHMEN136 *AP OF OUR SENIORS LIMIT 100% ARE ACCEPTED TO 2020-2021 PROFILE PER STUDENTS19 %OF COLOR 24APs 6STUDENT FOUR-YEAR COLLEGES Wesleyan’s mission is to be a Christian school of KENNETH CONNOR CHRIS CLEVELAND academic excellence by providing each student a Director of College Advising Head of School [email protected] diverse college preparatory education guided by 678-223-2281 RAMONA BLANKENSHIP Christian principles and beliefs; by challenging and Associate Head of School ANDREA SHUPERT nurturing the mind, body, and spirit; and by Assistant Director of JOSEPH KOCH developing responsible stewardship in our changing College Advising High School Principal world. Our school community believes in The Honor [email protected] 678-223-2116 5405 Spalding Dr. Code as a standard by which all members of our Peachtree Corners, GA 30092 school family are judged, respect is earned, and work ERICA ENGSBERG www.wesleyanschool.org Assistant Director of CEEB Code: 110261 is validated. College Advising [email protected] CURRICULUM MAP 678-223-2248 SUBJECT 9TH GRADE COURSES 10TH GRADE COURSES 11TH GRADE COURSES 12TH GRADE COURSES English (4) World Literature American Literature British Literature English Seminar World Literature H American Literature H British Literature H Literary Types/Composition AP Language and Composition AP Literature and Composition Mathematics (4) Algebra I Algebra II Geometry Algebra III Algebra II Geometry Pre-Calculus Calculus Algebra II H Geometry