Mechanisms of Differential Toxicity Between Honey Bee (<I>Apis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zootaxa,Phylogeny and Higher Classification of the Scale Insects
Zootaxa 1668: 413–425 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea)* P.J. GULLAN1 AND L.G. COOK2 1Department of Entomology, University of California, One Shields Avenue, Davis, CA 95616, U.S.A. E-mail: [email protected] 2School of Integrative Biology, The University of Queensland, Brisbane, Queensland 4072, Australia. Email: [email protected] *In: Zhang, Z.-Q. & Shear, W.A. (Eds) (2007) Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa, 1668, 1–766. Table of contents Abstract . .413 Introduction . .413 A review of archaeococcoid classification and relationships . 416 A review of neococcoid classification and relationships . .420 Future directions . .421 Acknowledgements . .422 References . .422 Abstract The superfamily Coccoidea contains nearly 8000 species of plant-feeding hemipterans comprising up to 32 families divided traditionally into two informal groups, the archaeococcoids and the neococcoids. The neococcoids form a mono- phyletic group supported by both morphological and genetic data. In contrast, the monophyly of the archaeococcoids is uncertain and the higher level ranks within it have been controversial, particularly since the late Professor Jan Koteja introduced his multi-family classification for scale insects in 1974. Recent phylogenetic studies using molecular and morphological data support the recognition of up to 15 extant families of archaeococcoids, including 11 families for the former Margarodidae sensu lato, vindicating Koteja’s views. Archaeococcoids are represented better in the fossil record than neococcoids, and have an adequate record through the Tertiary and Cretaceous but almost no putative coccoid fos- sils are known from earlier. -

Correlation of Stylet Activities by the Glassy-Winged Sharpshooter, Homalodisca Coagulata (Say), with Electrical Penetration Graph (EPG) Waveforms
ARTICLE IN PRESS Journal of Insect Physiology 52 (2006) 327–337 www.elsevier.com/locate/jinsphys Correlation of stylet activities by the glassy-winged sharpshooter, Homalodisca coagulata (Say), with electrical penetration graph (EPG) waveforms P. Houston Joosta, Elaine A. Backusb,Ã, David Morganc, Fengming Yand aDepartment of Entomology, University of Riverside, Riverside, CA 92521, USA bUSDA-ARS Crop Diseases, Pests and Genetics Research Unit, San Joaquin Valley Agricultural Sciences Center, 9611 South Riverbend Ave, Parlier, CA 93648, USA cCalifornia Department of Food and Agriculture, Mt. Rubidoux Field Station, 4500 Glenwood Dr., Bldg. E, Riverside, CA 92501, USA dCollege of Life Sciences, Peking Univerisity, Beijing, China Received 5 May 2005; received in revised form 29 November 2005; accepted 29 November 2005 Abstract Glassy-winged sharpshooter, Homalodisca coagulata (Say), is an efficient vector of Xylella fastidiosa (Xf), the causal bacterium of Pierce’s disease, and leaf scorch in almond and oleander. Acquisition and inoculation of Xf occur sometime during the process of stylet penetration into the plant. That process is most rigorously studied via electrical penetration graph (EPG) monitoring of insect feeding. This study provides part of the crucial biological meanings that define the waveforms of each new insect species recorded by EPG. By synchronizing AC EPG waveforms with high-magnification video of H. coagulata stylet penetration in artifical diet, we correlated stylet activities with three previously described EPG pathway waveforms, A1, B1 and B2, as well as one ingestion waveform, C. Waveform A1 occured at the beginning of stylet penetration. This waveform was correlated with salivary sheath trunk formation, repetitive stylet movements involving retraction of both maxillary stylets and one mandibular stylet, extension of the stylet fascicle, and the fluttering-like movements of the maxillary stylet tips. -

Twenty-Five Pests You Don't Want in Your Garden
Twenty-five Pests You Don’t Want in Your Garden Prepared by the PA IPM Program J. Kenneth Long, Jr. PA IPM Program Assistant (717) 772-5227 [email protected] Pest Pest Sheet Aphid 1 Asparagus Beetle 2 Bean Leaf Beetle 3 Cabbage Looper 4 Cabbage Maggot 5 Colorado Potato Beetle 6 Corn Earworm (Tomato Fruitworm) 7 Cutworm 8 Diamondback Moth 9 European Corn Borer 10 Flea Beetle 11 Imported Cabbageworm 12 Japanese Beetle 13 Mexican Bean Beetle 14 Northern Corn Rootworm 15 Potato Leafhopper 16 Slug 17 Spotted Cucumber Beetle (Southern Corn Rootworm) 18 Squash Bug 19 Squash Vine Borer 20 Stink Bug 21 Striped Cucumber Beetle 22 Tarnished Plant Bug 23 Tomato Hornworm 24 Wireworm 25 PA IPM Program Pest Sheet 1 Aphids Many species (Homoptera: Aphididae) (Origin: Native) Insect Description: 1 Adults: About /8” long; soft-bodied; light to dark green; may be winged or wingless. Cornicles, paired tubular structures on abdomen, are helpful in identification. Nymph: Daughters are born alive contain- ing partly formed daughters inside their bodies. (See life history below). Soybean Aphids Eggs: Laid in protected places only near the end of the growing season. Primary Host: Many vegetable crops. Life History: Females lay eggs near the end Damage: Adults and immatures suck sap from of the growing season in protected places on plants, reducing vigor and growth of plant. host plants. In spring, plump “stem Produce “honeydew” (sticky liquid) on which a mothers” emerge from these eggs, and give black fungus can grow. live birth to daughters, and theygive birth Management: Hide under leaves. -

Melon Aphid Or Cotton Aphid, Aphis Gossypii Glover (Insecta: Hemiptera: Aphididae)1 John L
EENY-173 Melon Aphid or Cotton Aphid, Aphis gossypii Glover (Insecta: Hemiptera: Aphididae)1 John L. Capinera2 Distribution generation can be completed parthenogenetically in about seven days. Melon aphid occurs in tropical and temperate regions throughout the world except northernmost areas. In the In the south, and at least as far north as Arkansas, sexual United States, it is regularly a pest in the southeast and forms are not important. Females continue to produce southwest, but is occasionally damaging everywhere. Be- offspring without mating so long as weather allows feeding cause melon aphid sometimes overwinters in greenhouses, and growth. Unlike many aphid species, melon aphid is and may be introduced into the field with transplants in the not adversely affected by hot weather. Melon aphid can spring, it has potential to be damaging almost anywhere. complete its development and reproduce in as little as a week, so numerous generations are possible under suitable Life Cycle and Description environmental conditions. The life cycle differs greatly between north and south. In the north, female nymphs hatch from eggs in the spring on Egg the primary hosts. They may feed, mature, and reproduce When first deposited, the eggs are yellow, but they soon parthenogenetically (viviparously) on this host all summer, become shiny black in color. As noted previously, the eggs or they may produce winged females that disperse to normally are deposited on catalpa and rose of sharon. secondary hosts and form new colonies. The dispersants typically select new growth to feed upon, and may produce Nymph both winged (alate) and wingless (apterous) female The nymphs vary in color from tan to gray or green, and offspring. -

Lifetime Organophosphorous Insecticide Use Among Private Pesticide Applicators in the Agricultural Health Study
Journal of Exposure Science and Environmental Epidemiology (2012) 22, 584 -- 592 & 2012 Nature America, Inc. All rights reserved 1559-0631/12 www.nature.com/jes ORIGINAL ARTICLE Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study Jane A. Hoppin1, Stuart Long2, David M. Umbach3, Jay H. Lubin4, Sarah E. Starks5, Fred Gerr5, Kent Thomas6, Cynthia J. Hines7, Scott Weichenthal8, Freya Kamel1, Stella Koutros9, Michael Alavanja9, Laura E. Beane Freeman9 and Dale P. Sandler1 Organophosphorous insecticides (OPs) are the most commonly used insecticides in US agriculture, but little information is available regarding specific OP use by individual farmers. We describe OP use for licensed private pesticide applicators from Iowa and North Carolina in the Agricultural Health Study (AHS) using lifetime pesticide use data from 701 randomly selected male participants collected at three time periods. Of 27 OPs studied, 20 were used by 41%. Overall, 95% had ever applied at least one OP. The median number of different OPs used was 4 (maximum ¼ 13). Malathion was the most commonly used OP (74%) followed by chlorpyrifos (54%). OP use declined over time. At the first interview (1993--1997), 68% of participants had applied OPs in the past year; by the last interview (2005--2007), only 42% had. Similarly, median annual application days of OPs declined from 13.5 to 6 days. Although OP use was common, the specific OPs used varied by state, time period, and individual. Much of the variability in OP use was associated with the choice of OP, rather than the frequency or duration of application. -

Biodetoxification of Coumaphos Insecticide Using Immobilized Escherichia Coli Expressing Organophosphorus Hydrolase Enzyme on Cell Surface
Biotechnol. Bioprocess Eng. 2000, 5: 436-440 Biodetoxification of Coumaphos Insecticide Using Immobilized Escherichia coli Expressing Organophosphorus Hydrolase Enzyme on Cell Surface Ayman H. Mansee2, Wilfred Chen1, and Ashok Mulchandani1* 1 Department of Chemical and Environmental Engineering, University of California, Riverside, CA 92521, USA 2 On leave from Pesticide Chemistry Department, Faculty of Agriculture (El-Shatby), Alexandria University, Alexandria, Egypt Abstract Recently, we reported an improved technology for the degradation of organophos- phate nerve agents using whole cells of genetically engineered Escherichia coli that anchored and displayed the enzyme organophosphorus hydrolase on the cell surface. In this paper we report the immobilization of these cells on highly porous sintered glass beads and the subse- quent application of the immobilized cell in a continuous-flow packed bed bioreactor for the biodetoxification of a widely used insecticide, coumaphos. Keywords: OPH, pesticides, nerve agents, bioreactor, porous sintered glass beads INTRODUCTION (APHIS) operates a Tick Eradication Program designed to prevent the re-introduction of Cattle Fever into the Neurotoxic organophosphates (OPs), amongst the United States through ticks (Boophilus microplus and most toxic compound known, are widely used as pesti- Boophilus annulatus) on cattle imported from Mexico. cides, insecticides and chemical warfare agents. In the The primary tool used in the eradication program is a United States annually over 40 million kg of organo- series of dipping vats placed at the border crossing phosphate pesticides are consumed [1] while another 20 points. Cattle coming in from Mexico must be dipped million kg are produced for export [2]. Chlorpyrifos in vats before entering the US. Currently, coumaphos is (lorsban) and parathion are two of most popular pesti- the pesticide of choice for this application. -

Establishment of the Brown Citrus Aphid Leadership of David Gumpf, UC University of Wisconsin, Madison; E.E
mation on ELB, the goals of the pro- Invisible invaders . gram and specifics on the treatments in their area. STEP also made presen- tations to neighborhood associations and displayed an information table at Insect-t ransmitted viruses a popular outdoor market throughout the summer. Through community education and outreach, the coopera- threaten agriculture tion of city agencies, good monitoring and the development of new strategies Robert L. Gilbertson a Diane E. Ullman, first authors to control elm leaf beetles in ”hot Raquel Salati 3 Douglas P. Maxwell spots,” the IPM program for Sacra- mento is being developed and should Elizabeth E. Grafton-Cardwell o MaryLou Polek be fully implemented within several years. The vast movement of people and Despite technological advances lead- D.L. Dahlsten is Professor and Associate agricultural products between dis- ing to tremendous yield increases for Dean, D.L. Rowney is Biostatistician and tant geographical regions has cre- many crops, modern agricultural pro- A.B. Lazuson is Graduate Student Re- ated unprecedented opportunities duction continues to face pest threats, searcher, Center for Biological Control, for introducing plant viruses and among them insects, plant pathogens, Division of Insect Biology, College of the insects that carry them (vec- and weeds. Often growers are faced Natural Resources, UC Berkeley. tors) to new areas. Outbreaks of with multiple pests, which exacerbates The authors thank the many individu- new viruses may be favored in crop damage and complicates manage- als and agencies that have assisted in the these agroecosystems by crop ment strategies. A dramatic example development of this program over the of how two types of pests “team up” susceptibility, the presence of par- years, and particularly Steve Dreistadt, to cause major problems for California UC Davis, UC/IPM Project; Beverly ticular weeds and certain agricul- agricultural production is the case of Gingg, San Luis Obispo; Anne Fenkner tural practices. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

The Leafhopper Vectors of Phytopathogenic Viruses (Homoptera, Cicadellidae) Taxonomy, Biology, and Virus Transmission
/«' THE LEAFHOPPER VECTORS OF PHYTOPATHOGENIC VIRUSES (HOMOPTERA, CICADELLIDAE) TAXONOMY, BIOLOGY, AND VIRUS TRANSMISSION Technical Bulletin No. 1382 Agricultural Research Service UMTED STATES DEPARTMENT OF AGRICULTURE ACKNOWLEDGMENTS Many individuals gave valuable assistance in the preparation of this work, for which I am deeply grateful. I am especially indebted to Miss Julianne Rolfe for dissecting and preparing numerous specimens for study and for recording data from the literature on the subject matter. Sincere appreciation is expressed to James P. Kramer, U.S. National Museum, Washington, D.C., for providing the bulk of material for study, for allowing access to type speci- mens, and for many helpful suggestions. I am also grateful to William J. Knight, British Museum (Natural History), London, for loan of valuable specimens, for comparing type material, and for giving much useful information regarding the taxonomy of many important species. I am also grateful to the following persons who allowed me to examine and study type specimens: René Beique, Laval Univer- sity, Ste. Foy, Quebec; George W. Byers, University of Kansas, Lawrence; Dwight M. DeLong and Paul H. Freytag, Ohio State University, Columbus; Jean L. LaiFoon, Iowa State University, Ames; and S. L. Tuxen, Universitetets Zoologiske Museum, Co- penhagen, Denmark. To the following individuals who provided additional valuable material for study, I give my sincere thanks: E. W. Anthon, Tree Fruit Experiment Station, Wenatchee, Wash.; L. M. Black, Uni- versity of Illinois, Urbana; W. E. China, British Museum (Natu- ral History), London; L. N. Chiykowski, Canada Department of Agriculture, Ottawa ; G. H. L. Dicker, East Mailing Research Sta- tion, Kent, England; J. -

Recent Advances on Detection of Insecticides Using Optical Sensors
sensors Review Recent Advances on Detection of Insecticides Using Optical Sensors Nurul Illya Muhamad Fauzi 1, Yap Wing Fen 1,2,*, Nur Alia Sheh Omar 1,2 and Hazwani Suhaila Hashim 2 1 Functional Devices Laboratory, Institute of Advanced Technology, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] (N.I.M.F.); [email protected] (N.A.S.O.) 2 Department of Physics, Faculty of Science, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] * Correspondence: [email protected] Abstract: Insecticides are enormously important to industry requirements and market demands in agriculture. Despite their usefulness, these insecticides can pose a dangerous risk to the safety of food, environment and all living things through various mechanisms of action. Concern about the environmental impact of repeated use of insecticides has prompted many researchers to develop rapid, economical, uncomplicated and user-friendly analytical method for the detection of insecticides. In this regards, optical sensors are considered as favorable methods for insecticides analysis because of their special features including rapid detection time, low cost, easy to use and high selectivity and sensitivity. In this review, current progresses of incorporation between recognition elements and optical sensors for insecticide detection are discussed and evaluated well, by categorizing it based on insecticide chemical classes, including the range of detection and limit of detection. Additionally, this review aims to provide powerful insights to researchers for the future development of optical sensors in the detection of insecticides. Citation: Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Hashim, H.S. Recent Keywords: insecticides; optical sensor; recognition element Advances on Detection of Insecticides Using Optical Sensors. -
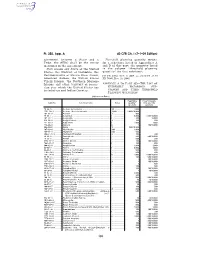
40 CFR Ch. I (7–1–09 Edition) Pt. 355, App. A
Pt. 355, App. A 40 CFR Ch. I (7–1–09 Edition) agreement between a State and a Threshold planning quantity means, Tribe, the SERC shall be the entity for a substance listed in Appendices A identified in the agreement. and B of this part, the quantity listed State means any State of the United in the column ‘‘threshold planning States, the District of Columbia, the quantity’’ for that substance. Commonwealth of Puerto Rico, Guam, [73 FR 65462, Nov. 3, 2008, as amended at 73 American Samoa, the United States FR 76960, Dec. 18, 2008] Virgin Islands, the Northern Mariana Islands, any other territory or posses- APPENDIX A TO PART 355—THE LIST OF sion over which the United States has EXTREMELY HAZARDOUS SUB- jurisdiction and Indian Country. STANCES AND THEIR THRESHOLD PLANNING QUANTITIES [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 75–86–5 ................ Acetone Cyanohydrin ................................................. 10 ................ 1,000 1752–30–3 ............ Acetone Thiosemicarbazide ....................................... 1,000 ........... 1,000/10,000 107–02–8 .............. Acrolein ....................................................................... 1 .................. 500 79–06–1 ................ Acrylamide .................................................................. f ................... 5,000 1,000/10,000 107–13–1 .............. Acrylonitrile ................................................................. f ................... 100 10,000 -

Biosecurity Risk Assessment
An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries RIRDC Publication No. 11/141 RIRDCInnovation for rural Australia An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries by Dr Robert C Keogh February 2012 RIRDC Publication No. 11/141 RIRDC Project No. PRJ-007347 © 2012 Rural Industries Research and Development Corporation. All rights reserved. ISBN 978-1-74254-320-8 ISSN 1440-6845 An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries Publication No. 11/141 Project No. PRJ-007347 The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. The Commonwealth of Australia does not necessarily endorse the views in this publication.