Middle Segment Pancreatectomy for a Solid Serous Cystadenoma Diagnosed by MRCP and Review of the Literature: a Case Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OT Resource for K9 Overview of Surgical Procedures
OT Resource for K9 Overview of surgical procedures Prepared by: Hannah Woolley Stage Level 1 2 Gynecology/Oncology Surgeries Lymphadenectomy (lymph node dissection) Surgical removal of lymph nodes Radical: most/all of the lymph nodes in tumour area are removed Regional: some of the lymph nodes in the tumour area are removed Omentectomy Surgical procedure to remove the omentum (thin abdominal tissue that encases the stomach, large intestine and other abdominal organs) Indications for omenectomy: Ovarian cancer Sometimes performed in combination with TAH/BSO Posterior Pelvic Exenteration Surgical removal of rectum, anus, portion of the large intestine, ovaries, fallopian tubes and uterus (partial or total removal of the vagina may also be indicated) Indications for pelvic exenteration Gastrointestinal cancer (bowel, colon, rectal) Gynecological cancer (cervical, vaginal, ovarian, vulvar) Radical Cystectomy Surgical removal of the whole bladder and proximal lymph nodes In men, prostate gland is also removed In women, ovaries and uterus may also be removed Following surgery: Urostomy (directs urine through a stoma on the abdomen) Recto sigmoid pouch/Mainz II pouch (segment of the rectum and sigmoid colon used to provide anal urinary diversion) 3 Radical Vulvectomy Surgical removal of entire vulva (labia, clitoris, vestibule, introitus, urethral meatus, glands/ducts) and surrounding lymph nodes Indication for radical vulvectomy Treatment of vulvar cancer (most common) Sentinel Lymph Node Dissection (SLND) Exploratory procedure where the sentinel lymph node is removed and examined to determine if there is lymph node involvement in patients diagnosed with cancer (commonly breast cancer) Total abdominal hysterectomy/bilateral saplingo-oophorectomy (TAH/BSO) Surgical removal of the uterus (including cervix), both fallopian tubes and ovaries Indications for TAH/BSO: Uterine fibroids: benign growths in the muscle of the uterus Endometriosis: condition where uterine tissue grows on structures outside the uterus (i.e. -

Complication Analysis of Distal Pancreatectomy Based on Early Personal Experience
Korean J Hepatobiliary Pancreat Surg 2011;15:243-247 Original Article Complication analysis of distal pancreatectomy based on early personal experience Sung-Jin Park, Hyung-Il Seo, Soo-Hee Go, Sung-Pil Yun, and Ji-Yeon Lee Department of Surgery, Postgraduate School of Medicine, Pusan National University, Busan, Korea Backgrounds/Aims: The objective of this study was to evaluate the relationship between initial personal experiences with distal pancreatectomy and perioperative risk factors, outcomes, and management of pancreatic fistulas. Methods: Between May, 2007 and May, 2010, a total of 28 patients who had undergone elective distal pancreatectomy were evaluated for this study. Perioperative factors and the occurrence of pancreatic fistula were analyzed on the basis of International Study Group of Pancreatic Fistula (ISGPF) criteria. Results: There were sixteen cases of benign neo- plasms and twelve cases of malignant tumors. The remnant pancreas was manually sutured with ligation of the pancre- atic duct (n=14), auto-suture stapling along with manual sutures (n=12), or stapling alone (n=2). According to the ISGPF classification, morbidity and mortality associated with pancreatic fistulas was 42.9% (n=12) and 0%, respectively. These pancreatic fistulae were classified as grade A in 8 cases (28.6%), grade B in 3 cases (10.7%), and grade C in one case (3.6%). All patients with pancreatic fistula were treated conservatively. Conclusions: Perioperative factors do not affect the risk of pancreatic fistula. Adequate drainage is the most effective method for management of a pancreatic fistula after distal pancreatectomy. (Korean J Hepatobiliary Pancreat Surg 2011;15:243-247) Key Words: Distal pancreatectomy; Pancreatic fistula; Complication; Leak INTRODUCTION Study Group for Pancreatic Fistula (ISGPF). -

Chemical Pancreatectomy Treats Chronic Pancreatitis While Preserving Endocrine Function in Preclinical Models
Chemical pancreatectomy treats chronic pancreatitis while preserving endocrine function in preclinical models Mohamed Saleh, … , Krishna Prasadan, George Gittes J Clin Invest. 2020. https://doi.org/10.1172/JCI143301. Research In-Press Preview Endocrinology Gastroenterology Chronic pancreatitis affects over 250,000 people in the US and millions worldwide. It is associated with chronic debilitating pain, pancreatic exocrine failure, high-risk of pancreatic cancer, and usually progresses to diabetes. Treatment options are limited and ineffective. We developed a new potential therapy, wherein a pancreatic ductal infusion of 1-2% acetic acid in mice and non-human primates resulted in a non-regenerative, near-complete ablation of the exocrine pancreas, with complete preservation of the islets. Pancreatic ductal infusion of acetic acid in a mouse model of chronic pancreatitis led to resolution of chronic inflammation and pancreatitis-associated pain. Furthermore, acetic acid-treated animals showed improved glucose tolerance and insulin secretion. The loss of exocrine tissue in this procedure would not typically require further management in patients with chronic pancreatitis because they usually have pancreatic exocrine failure requiring dietary enzyme supplements. Thus, this procedure, which should be readily translatable to humans through an endoscopic retrograde cholangiopancreatography (ERCP), may offer a potential innovative non-surgical therapy for chronic pancreatitis that relieves pain and prevents the progression of pancreatic diabetes. Find the latest version: https://jci.me/143301/pdf Chemical pancreatectomy treats chronic pancreatitis while preserving endocrine function in preclinical models Authors: Mohamed Saleh1,2, *Kartikeya Sharma1, *Ranjeet Kalsi1, Joseph Fusco1, Anuradha Sehrawat1, Jami L. Saloman3, Ping Guo4, Ting Zhang 1, Nada Mohamed1, Yan Wang1, Krishna Prasadan1, George K. -

Original Article
ABCD Arq Bras Cir Dig Original Article - Technique 2018;31(3):e1395 DOI: /10.1590/0102-672020180001e1395 LAPAROSCOPIC DISTAL PANCREATECTOMY WITH SPLEEN PRESERVATION Pancreatectomia distal laparoscópica com preservação esplênica Sergio Renato PAIS-COSTA1,2, Guilherme Costa Crispim de SOUSA1,2, Sergio Luiz Melo ARAUJO1,2, Olímpia Alves Teixeira LIMA1,2 How to cite this article: Pais-Costa SR, Sousa GCC, Araujo SLM, Lima OAT. Laparoscopic distal pancreatectomy with preservation of the spleen. ABCD Arq Bras Cir Dig. 2018;31(3):e1395. DOI: /10.1590/0102-672020180001e1395 From the 1Hospital Santa Lúcia, Brasília, DF ABSTRACT - Background: Laparoscopic distal pancreatectomy has been the choice for resection and 2Hospital Brasília, Brasília, DF, Brasil. of distal pancreas lesions due many advantages over open approach. Spleen preservation technique seems minimizes infectious complications in long-term outcome. Aim: To present the results of laparoscopic distal pancreatectomies with spleen preservation by Kimura´s technique (preservation of spleen blood vessels) performed by single surgical team. Methods: Retrospective case series aiming to evaluate both short and long-term outcomes of laparoscopic distal pancreatectomies with spleen preservation. Results: A total of 54 laparoscopic distal pancreatectomies were performed, in which 26 were laparoscopic distal pancreatectomies with spleen preservation by Kimura´s technique. Mean age was 47.9 years-old (21-75) where 61.5% were female. Mean BMI was 28.5 kg/m² (18-38.8). Mean diameter of lesion was 4.3 cm (1.8- 7.5). Mean operative time was 144.1 min (90-200). Intraoperative bleeding was 119.2 ml (50- 600). Conversion to laparotomy 3% (n=1). -
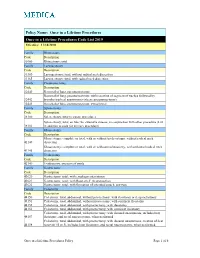
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Leapfrog Hospital Survey Hard Copy
Leapfrog Hospital Survey Hard Copy QUESTIONS & REPORTING PERIODS ENDNOTES MEASURE SPECIFICATIONS FAQS Table of Contents Welcome to the 2016 Leapfrog Hospital Survey........................................................................................... 6 Important Notes about the 2016 Survey ............................................................................................ 6 Overview of the 2016 Leapfrog Hospital Survey ................................................................................ 7 Pre-Submission Checklist .................................................................................................................. 9 Instructions for Submitting a Leapfrog Hospital Survey ................................................................... 10 Helpful Tips for Verifying Submission ......................................................................................... 11 Tips for updating or correcting a previously submitted Leapfrog Hospital Survey ...................... 11 Deadlines ......................................................................................................................................... 13 Deadlines for the 2016 Leapfrog Hospital Survey ...................................................................... 13 Deadlines Related to the Hospital Safety Score ......................................................................... 13 Technical Assistance....................................................................................................................... -

Surgical Resection of Hepatic and Rectal Metastases of Pancreatic Acinar Cell Carcinoma (PACC): a Case Report
Surgical resection of hepatic and rectal metastases of pancreatic acinar cell carcinoma (PACC): a case report 著者 Ohara Yusuke, Oda Tatsuya, Enomoto Tsuyoshi, Hisakura Katsuji, Akashi Yoshimasa, Ogawa Koichi, Owada Yohei, Domoto Yu, Miyazaki Yoshihiro, Shimomura Osamu, Kurata Masanao, Ohkohchi Nobuhiro journal or World journal of surgical oncology publication title volume 16 page range 158 year 2018-08 権利 (C) The Author(s). 2018 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1 .0/ ) applies to the data made available in this article, unless otherwise stated. URL http://hdl.handle.net/2241/00153588 doi: 10.1186/s12957-018-1457-8 Creative Commons : 表示 http://creativecommons.org/licenses/by/3.0/deed.ja Ohara et al. World Journal of Surgical Oncology (2018) 16:158 https://doi.org/10.1186/s12957-018-1457-8 CASE REPORT Open Access Surgical resection of hepatic and rectal metastases of pancreatic acinar cell carcinoma (PACC): a case report Yusuke Ohara, Tatsuya Oda*, Tsuyoshi Enomoto, Katsuji Hisakura, Yoshimasa Akashi, Koichi Ogawa, Yohei Owada, Yu Domoto, Yoshihiro Miyazaki, Osamu Shimomura, Masanao Kurata and Nobuhiro Ohkohchi Abstract Background: Pancreatic acinar cell carcinoma (PACC), a rare variant of pancreatic malignancy, is generally managed the same way as pancreatic ductal adenocarcinoma (PDAC). -

Laparoscopic Hand-Assisted Total Pancreatectomy: Single Institution Experience of Seven Patients
JOP. J Pancreas (Online) 2020 June 30; 21(3): 57-62. ORIGINAL ARTICLE Laparoscopic Hand-assisted Total Pancreatectomy: Single Institution Experience of Seven Patients Sujit Kulkarni1, Kaylene Barrera2, Rick Selby1, Dilipkumar Parekh1 1Department of Surgery, Keck Medical Center, University of Southern California, Los Angeles, United States 2SUNY Downstate Medical Center, New York City, New York, United States ABSTRACT Background In the past two decades, total pancreatectomy has been associated with improved postoperative and long-term outcomes due to the improvements in surgical technique, better enzyme preparations and diabetes control. While minimally invasive Whipple operation has enjoyed the attention in recent years, the safety and feasibility of a minimally invasive total pancreatectomy is still not established. Methods A retrospective review of minimally invasive total pancreatic resections. Results Seven patients underwent laparoscopic hand- assisted total pancreatectomy between 2005 and 2011. The mean patient age was 58.1 years (58.1 ± 6.45) and the median American Society of Anesthesiologist score was 3. Three patients had diffuse IPMN, two had multiple neuroendocrine tumors and two patients had large cystic lesions in head, body and tail of pancreas. Median operative time was 431 minutes (range 348-590) with 300 cc (range the mortality was 0. Conclusion The laparoscopic hand-assisted total pancreatectomy appears to be a safe and feasible procedure. It is a150-1200) technically of demanding blood loss. procedureThe 90 days requiring postoperative expertise complication in both open rate and of advancedgrade 2 or laparoscopic higher Clavien-Dindo pancreatic classification procedures andwas additional 14% and multi-institutional studies are necessary to further evaluate its role. -

The Evaluation of the Early and Late Postoperative Pancreatic Function and Nutritional Status: Central Pancreatectomy Versus Distal Pancreatectomy
ISSN: 2378-3397 Izumo et al. Int J Surg Res Pract 2017, 4:057 DOI: 10.23937/2378-3397/1410057 Volume 4 | Issue 3 International Journal of Open Access Surgery Research and Practice RESEARCH ARTICLE The Evaluation of the Early and Late Postoperative Pancreatic Func- tion and Nutritional Status: Central Pancreatectomy Versus Distal Pancreatectomy Wataru Izumo*, Ryota Higuchi and Masakazu Yamamoto Department of Surgery, Institute of Gastroenterology, Tokyo Women’s Medical University, Japan *Corresponding author: Wataru Izumo, Department of Surgery, Institute of Gastroenterology, Tokyo Women’s Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo 162-8666, Japan, E-mail: [email protected]; [email protected] Abstract Introduction Background: Central pancreatectomy is performed to The pancreas has both exocrine and endocrine func- preserve pancreatic function in selected patients with low- tions, and plays an important role in digestion and ab- grade tumors. We evaluated short-term and long-term pan- sorption by secreting pancreatic digestive enzymes in creatic function and nutritional status after central or distal pancreatectomy. the pancreatic juice and releasing insulin into the blood. In recent years, central pancreatectomy has been per- Methods: The subjects were 24 patients undergoing central pancreatectomy and 91 patients receiving distal pancrea- formed to preserve pancreatic function in selected pa- tectomy. We retrospectively evaluated body weight, serum tients with low-grade pancreatic tumors, but its short- albumin, hemoglobin A1c, and complications. term and long-term efficacy for achieving this objective Results: After central pancreatectomy, body weight and he- has been not so clear. moglobin A1c did not change significantly up to 60 months postoperatively compared with before surgery, while serum Theoretically, pancreatic exocrine function should albumin was significantly increased at all postoperative as- be better after central pancreatectomy than after other sessments (6, 12, 36, and 60 months, all P < 0.05). -

Ex Vivo Resection and Autotransplantation for Pancreatic Neoplasms
Ex Vivo Resection and Autotransplantation for Pancreatic Neoplasms Peter Liou, MD, Tomoaki Kato, MD, MBA* KEYWORDS Pancreas Pancreatic tumors Ex vivo resection Autotransplantation Mesenteric root involvement SMA involvement KEY POINTS Ex vivo resection and autotransplantation is a technique derived from multivisceral and intestinal transplantation whereby tumor-infiltrated organs are removed en bloc and pre- served in the cold, followed by tumor resection and reimplantation of the remaining viscera. Advantages of ex vivo resection include tumor removal in a bloodless field while mini- mizing the risk of ischemic injury to the involved organs. Access to the mesenteric root is greatly facilitated with ex vivo resection, and allows for safe reconstruction of major vasculature while preserving visceral integrity. Certain low-grade, non-adenocarcinomatous pancreatic neoplasms involving the mesen- teric vessels where aggressive surgical resection would be warranted, may benefit from ex vivo resection. Although ex vivo resections have been performed for pancreatic adenocarcinomas with major arterial involvement, the associated morbidity is significant and benefit remains unclear. INTRODUCTION Pancreatic neoplasms are a heterogeneous group of tumors arising from the pancreas with distinct and varied clinical profiles.1 Although pancreatic adenocarcinoma remains by far the most common and deadliest of these, there are several low- grade or benign neoplasms that may benefit from aggressive, curative resection.2,3 Due to the proximity of the pancreas to major abdominal vasculature, these tumors can sometimes infiltrate these vessels and preclude complete or safe resection by conventional surgical technique. Ex vivo resection and autotransplantation, whereby Financial Disclosures: The authors have nothing to disclose. Department of Surgery, Columbia University Medical Center, 622 West 168 Street PH14-105, New York, NY 10032, USA * Corresponding author. -

Post-Whipple: a Practical Approach to Nutrition Management
NINFLAMMATORYUTRITION ISSUES BOWEL IN GASTROENTEROLO DISEASE: A PRACTICALGY, SERIES APPROACH, #108 SERIES #73 Carol Rees Parrish, M.S., R.D., Series Editor Post-Whipple: A Practical Approach to Nutrition Management Nora Decher Amy Berry The classic pancreatoduodenectomy (PD), also known as the Kausch-Whipple, and the pylorus- preserving pancreatoduodenectomy (PPPD) are most commonly performed for treatment of pancreatic cancer and chronic pancreatitis. This highly complex surgery disrupts the coordination of tightly orchestrated digestive processes. This, in combination with a diseased gland, sets the patient up for nutritional complications such as altered motility (gastroparesis and dumping), pancreatic insufficiency, diabetes mellitus, nutritional deficiencies and bacterial overgrowth. Close monitoring and attention to these issues will help the clinician optimize nutritional status and help prevent potentially devastating complications. 63-year-old female, D.D., presented to the copper, zinc, selenium, and potentially thiamine University of Virginia Health System (UVAHS) (thiamine was repleted before serum levels were Awith weight loss and biliary obstruction. She was checked). A percutaneous endoscopic gastrostomy with diagnosed with a large pancreatic serous cystadenoma jejunal extension (PEG-J) was placed due to intolerance and underwent a pancreatoduodenectomy (PD) of gastric enteral nutrition (EN). After several more (standard Whipple procedure with partial gastrectomy) hospitalizations, prolonged rehabilitation in a nursing with posterior anastomosis and cholecystectomy. Seven home, 7 months of supplemental nocturnal EN, and months later she was admitted to UVAHS with nausea, treatment of pancreatic insufficiency with pancreatic vomiting, diarrhea and a severe weight loss of 47lbs enzymes (with her meals and EN), D.D. was able to (33% of her usual body weight). -
Facing Pancreatic Surgery?
Facing Pancreatic Surgery? Learn about minimally invasive da Vinci ® Surgery The Condition: Pancreatitis/Pancreatic Cancer The pancreas is an organ that produces enzymes and hormones to help your body digest food and regulate blood sugar. The pancreas is located behind your stomach and is about 6 inches long. Pancreatitis is a disease in which the pancreas becomes inflamed. It can be acute or chronic. Acute pancreatitis occurs suddenly and usually goes away in a few days with treatment.1 Symptoms may include: pain, swelling or tenderness in the upper abdomen, nausea, vomiting, back pain, and fever. Chronic pancreatitis occurs over many years and can lead to permanent damage.1 Symptoms may include: upper abdominal pain, nausea, vomiting, diarrhea, and oily stool. Pancreatitis often develops between ages 30 and 40. The most common causes are: gallstones, heavy alcohol use, cystic fibrosis, high triglycerides, certain medicines, and structural problems in the pancreas. Pancreatic cancer is hard to detect in its initial stages since it often does not cause early symptoms.2 If symptoms are present, they may include: yellowing skin or eyes, pain in the abdomen and back, loss of appetite, depression and blood clots. Some risk factors include: smoking, obesity, diabetes, chronic pancreatitis, and certain hereditary disorders. Stomach Pancreas Treatment/Surgery Your doctor will suggest a treatment plan based on the severity or stage of the disease. If surgery is recommended, the type of surgery (listed below) will depend on how much of your pancreas is affected. When cancer is suspected, your surgeon will remove your pancreas and send it to a lab to be tested for cancer.