Chemical Pancreatectomy Treats Chronic Pancreatitis While Preserving Endocrine Function in Preclinical Models
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OT Resource for K9 Overview of Surgical Procedures
OT Resource for K9 Overview of surgical procedures Prepared by: Hannah Woolley Stage Level 1 2 Gynecology/Oncology Surgeries Lymphadenectomy (lymph node dissection) Surgical removal of lymph nodes Radical: most/all of the lymph nodes in tumour area are removed Regional: some of the lymph nodes in the tumour area are removed Omentectomy Surgical procedure to remove the omentum (thin abdominal tissue that encases the stomach, large intestine and other abdominal organs) Indications for omenectomy: Ovarian cancer Sometimes performed in combination with TAH/BSO Posterior Pelvic Exenteration Surgical removal of rectum, anus, portion of the large intestine, ovaries, fallopian tubes and uterus (partial or total removal of the vagina may also be indicated) Indications for pelvic exenteration Gastrointestinal cancer (bowel, colon, rectal) Gynecological cancer (cervical, vaginal, ovarian, vulvar) Radical Cystectomy Surgical removal of the whole bladder and proximal lymph nodes In men, prostate gland is also removed In women, ovaries and uterus may also be removed Following surgery: Urostomy (directs urine through a stoma on the abdomen) Recto sigmoid pouch/Mainz II pouch (segment of the rectum and sigmoid colon used to provide anal urinary diversion) 3 Radical Vulvectomy Surgical removal of entire vulva (labia, clitoris, vestibule, introitus, urethral meatus, glands/ducts) and surrounding lymph nodes Indication for radical vulvectomy Treatment of vulvar cancer (most common) Sentinel Lymph Node Dissection (SLND) Exploratory procedure where the sentinel lymph node is removed and examined to determine if there is lymph node involvement in patients diagnosed with cancer (commonly breast cancer) Total abdominal hysterectomy/bilateral saplingo-oophorectomy (TAH/BSO) Surgical removal of the uterus (including cervix), both fallopian tubes and ovaries Indications for TAH/BSO: Uterine fibroids: benign growths in the muscle of the uterus Endometriosis: condition where uterine tissue grows on structures outside the uterus (i.e. -

Complication Analysis of Distal Pancreatectomy Based on Early Personal Experience
Korean J Hepatobiliary Pancreat Surg 2011;15:243-247 Original Article Complication analysis of distal pancreatectomy based on early personal experience Sung-Jin Park, Hyung-Il Seo, Soo-Hee Go, Sung-Pil Yun, and Ji-Yeon Lee Department of Surgery, Postgraduate School of Medicine, Pusan National University, Busan, Korea Backgrounds/Aims: The objective of this study was to evaluate the relationship between initial personal experiences with distal pancreatectomy and perioperative risk factors, outcomes, and management of pancreatic fistulas. Methods: Between May, 2007 and May, 2010, a total of 28 patients who had undergone elective distal pancreatectomy were evaluated for this study. Perioperative factors and the occurrence of pancreatic fistula were analyzed on the basis of International Study Group of Pancreatic Fistula (ISGPF) criteria. Results: There were sixteen cases of benign neo- plasms and twelve cases of malignant tumors. The remnant pancreas was manually sutured with ligation of the pancre- atic duct (n=14), auto-suture stapling along with manual sutures (n=12), or stapling alone (n=2). According to the ISGPF classification, morbidity and mortality associated with pancreatic fistulas was 42.9% (n=12) and 0%, respectively. These pancreatic fistulae were classified as grade A in 8 cases (28.6%), grade B in 3 cases (10.7%), and grade C in one case (3.6%). All patients with pancreatic fistula were treated conservatively. Conclusions: Perioperative factors do not affect the risk of pancreatic fistula. Adequate drainage is the most effective method for management of a pancreatic fistula after distal pancreatectomy. (Korean J Hepatobiliary Pancreat Surg 2011;15:243-247) Key Words: Distal pancreatectomy; Pancreatic fistula; Complication; Leak INTRODUCTION Study Group for Pancreatic Fistula (ISGPF). -

Criteria for Critical Care of Infants and Children: PICU Admission, Discharge, and Triage Practice Statement and Levels of Care Guidance Benson S
POLICY STATEMENT Organizational Principles to Guide and Define the Child Health Care System and/or Improve the Health of all Children Executive Summary: Criteria for Critical Care of Infants and Children: PICU Admission, Discharge, and Triage Practice Statement and Levels of Care Guidance Benson S. Hsu, MD, MBA, FAAP,a Vanessa Hill, MD, FAAP,b Lorry R. Frankel, MD, FCCM,c Timothy S. Yeh, MD, MCCM,d Shari Simone, CRNP, DNP, FCCM, FAANP, FAAN,e Marjorie J. Arca, MD, FACS, FAAP,f Jorge A. Coss-Bu, MD,g Mary E. Fallat, MD, FACS, FAAP,h Jason Foland, MD,i Samir Gadepalli, MD, MBA,j Michael O. Gayle, BS, MD, FCCM,k Lori A. Harmon, RRT, MBA, CPHQ,l Christa A. Joseph, RN, MSN,m Aaron D. Kessel, BS, MD,n Niranjan Kissoon, MD, MCCM,o Michele Moss, MD, FCCM,p Mohan R. Mysore, MD, FAAP, FCCM,q MicheleC . Papo, MD, MPH, FCCM,r Kari L. Rajzer-Wakeham, CCRN, MSN, PCCNP, RN,s Tom B. Rice, MD,t David L. Rosenberg, MD, FAAP, FCCM,u Martin K. Wakeham, MD,v,t Edward E. Conway, Jr, MD, FCCM, MS,w Michael S.D. Agus, MD, FAAP, FCCMx This is an executive summary of the 2019 update of the 2004 guidelines and levels of abstract care for PICU. Since previous guidelines, there has been a tremendous a transformation of Pediatric Critical Care Medicine with advancements in pediatric Pediatric Critical Care, Sanford School of Medicine, University of South Dakota, Vermillion, South Dakota; bHospital Medicine, Baylor cardiovascular medicine, transplant, neurology, trauma, and oncology as well as College of Medicine and Children’s Hospital of San Antonio, San improvements of care in general PICUs. -

Care of the Pediatric Patient in Surgery: Neonatal Through Adolescence ”
CARE OF THE PEDIATRIC PATIENT IN SURGERY : NEONATAL THROUGH ADOLESCENCE 1961 1961 CARE OF THE PEDIATRIC PATIENT IN SURGERY : NEONATAL THROUGH ADOLESCENCE STUDY GUIDE Disclaimer AORN and its logo are registered trademarks of AORN, Inc. AORN does not endorse any commercial company’s products or services. Although all commercial products in this course are expected to conform to professional medical/nursing standards, inclusion in this course does not constitute a guarantee or endorsement by AORN of the quality or value of such products or of the claims made by the manufacturers. No responsibility is assumed by AORN, Inc, for any injury and/or damage to persons or property as a matter of product liability, negligence or otherwise, or from any use or operation of any standards, recommended practices, methods, products, instructions, or ideas contained in the material herein. Because of rapid advances in the health care sciences in particular, independent verification of diagnoses, medication dosages, and individualized care and treatment should be made. The material contained herein is not intended to be a substitute for the exercise of professional medical or nursing judgment. The content in this publication is provided on an “as is” basis. TO THE FULLEST EXTENT PERMITTED BY LAW, AORN, INC, DISCLAIMS ALL WARRANTIES, EITHER EXPRESSED OR IMPLIED, STATUTORY OR OTHERWISE, INCLUDING BUT NOT LIMITED TO THE IMPLIED WARRANTIES OF MERCHANTABILITY, NON-INFRINGEMENT OF THIRD PARTIES’ RIGHTS, AND FITNESS FOR A PARTICULAR PURPOSE. This publication may be photocopied for noncommercial purposes of scientific use or educational advancement. The following credit line must appear on the front page of the photocopied document: Reprinted with permission from The Association of periOperative Registered Nurses, Inc. -

Original Article
ABCD Arq Bras Cir Dig Original Article - Technique 2018;31(3):e1395 DOI: /10.1590/0102-672020180001e1395 LAPAROSCOPIC DISTAL PANCREATECTOMY WITH SPLEEN PRESERVATION Pancreatectomia distal laparoscópica com preservação esplênica Sergio Renato PAIS-COSTA1,2, Guilherme Costa Crispim de SOUSA1,2, Sergio Luiz Melo ARAUJO1,2, Olímpia Alves Teixeira LIMA1,2 How to cite this article: Pais-Costa SR, Sousa GCC, Araujo SLM, Lima OAT. Laparoscopic distal pancreatectomy with preservation of the spleen. ABCD Arq Bras Cir Dig. 2018;31(3):e1395. DOI: /10.1590/0102-672020180001e1395 From the 1Hospital Santa Lúcia, Brasília, DF ABSTRACT - Background: Laparoscopic distal pancreatectomy has been the choice for resection and 2Hospital Brasília, Brasília, DF, Brasil. of distal pancreas lesions due many advantages over open approach. Spleen preservation technique seems minimizes infectious complications in long-term outcome. Aim: To present the results of laparoscopic distal pancreatectomies with spleen preservation by Kimura´s technique (preservation of spleen blood vessels) performed by single surgical team. Methods: Retrospective case series aiming to evaluate both short and long-term outcomes of laparoscopic distal pancreatectomies with spleen preservation. Results: A total of 54 laparoscopic distal pancreatectomies were performed, in which 26 were laparoscopic distal pancreatectomies with spleen preservation by Kimura´s technique. Mean age was 47.9 years-old (21-75) where 61.5% were female. Mean BMI was 28.5 kg/m² (18-38.8). Mean diameter of lesion was 4.3 cm (1.8- 7.5). Mean operative time was 144.1 min (90-200). Intraoperative bleeding was 119.2 ml (50- 600). Conversion to laparotomy 3% (n=1). -

PEDIATRIC SURGERY WELCOME to PEDIATRIC SURGERY This Is a Busy Surgical Service with Lots to See and Do
Medical Students Guide to PEDIATRIC SURGERY WELCOME TO PEDIATRIC SURGERY This is a busy surgical service with lots to see and do. Our goal is to integrate you into the service so that you can get the best experience possible during your time here. This guide details the structure of our service along with some pointers to help get you up and running. DAILY SCHEDULE 6 a.m. Morning rounds * 6th fl, East, NICU front desk 7 a.m. Radiology rounds 2nd fl, East, Radiology Body reading room 7:30 a.m. OR start (starts at 8:30 a.m. on Thurs) 2nd fl, Main 8 a.m. - 4 p.m. Clinic 4th fl Main, Suite 4400 5 p.m. Afternoon rounds 5th fl, East (outside room 501) *Sat & Sun rounds start at 7 or 7:30 a.m., confirm time with team ROTATION SCHEDULE Your rotation will start on August 31 and will end on September 18, 2020. See attached schedule. TEACHING SESSIONS: General surgery clinic days are Mondays, 1 Tuesdays, Thursdays and Fridays in Suite 4400 7:00 – 9:00 A.M. Telehealth clinics are generally scheduled at Thursday 2 the end of clinic Morning Pediatric Surgery Conference Guzzetta Library General surgery elective OR days by faculty 3 members are each day. 3:00 – 5:30 P.M. generally between Tuesdays and Fridays 4 The add-on room runs each day. Medical student lectures Guzzetta Library Your schedule will include time spent in the 5 surgery clinic, in the OR, with the consult resident, and with the surgeon of the day. -
Pediatric Surgery What You Need to Know
Pediatric Surgery What you need to know Norton Women’s & Children’s Hospital Welcome to the Pediatric Billing Surgery Center Most bills are submitted to your insurance company for payment. Our registration staff will ask you for your Norton Women’s & Children’s Hospital insurance information. Our staff of pediatric health care professionals understands the specialized care that is so important for children. If for some reason we are unable to submit claims to your Learning about your child’s surgery will help you and your insurance company for you, we will inform you immediately. child feel less anxious. In this instance, we will offer you all the necessary information you need regarding the different payment plans We’ve developed this brochure to answer some of your available to make the process as easy as possible. questions and give you important information before bringing your child to the Pediatric Surgery Center. Please After your child’s procedure is scheduled, attempts are read it carefully. It is important to us that your child has a made to verify your insurance coverage and copayment. It safe and pleasant surgical experience. is your responsibility to pay the copayment amount. There are many different kinds of insurance coverage, and some We know that no procedure is small or routine when it plans cover more than others. You may want to check with comes to your child. We consider you and your child as your insurance company regarding the coverage provided essential members of the health care team, and we promise by your individual plan. -
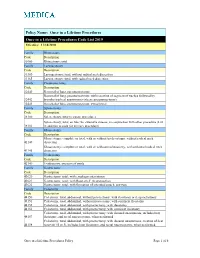
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Leapfrog Hospital Survey Hard Copy
Leapfrog Hospital Survey Hard Copy QUESTIONS & REPORTING PERIODS ENDNOTES MEASURE SPECIFICATIONS FAQS Table of Contents Welcome to the 2016 Leapfrog Hospital Survey........................................................................................... 6 Important Notes about the 2016 Survey ............................................................................................ 6 Overview of the 2016 Leapfrog Hospital Survey ................................................................................ 7 Pre-Submission Checklist .................................................................................................................. 9 Instructions for Submitting a Leapfrog Hospital Survey ................................................................... 10 Helpful Tips for Verifying Submission ......................................................................................... 11 Tips for updating or correcting a previously submitted Leapfrog Hospital Survey ...................... 11 Deadlines ......................................................................................................................................... 13 Deadlines for the 2016 Leapfrog Hospital Survey ...................................................................... 13 Deadlines Related to the Hospital Safety Score ......................................................................... 13 Technical Assistance....................................................................................................................... -

Surgical Resection of Hepatic and Rectal Metastases of Pancreatic Acinar Cell Carcinoma (PACC): a Case Report
Surgical resection of hepatic and rectal metastases of pancreatic acinar cell carcinoma (PACC): a case report 著者 Ohara Yusuke, Oda Tatsuya, Enomoto Tsuyoshi, Hisakura Katsuji, Akashi Yoshimasa, Ogawa Koichi, Owada Yohei, Domoto Yu, Miyazaki Yoshihiro, Shimomura Osamu, Kurata Masanao, Ohkohchi Nobuhiro journal or World journal of surgical oncology publication title volume 16 page range 158 year 2018-08 権利 (C) The Author(s). 2018 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1 .0/ ) applies to the data made available in this article, unless otherwise stated. URL http://hdl.handle.net/2241/00153588 doi: 10.1186/s12957-018-1457-8 Creative Commons : 表示 http://creativecommons.org/licenses/by/3.0/deed.ja Ohara et al. World Journal of Surgical Oncology (2018) 16:158 https://doi.org/10.1186/s12957-018-1457-8 CASE REPORT Open Access Surgical resection of hepatic and rectal metastases of pancreatic acinar cell carcinoma (PACC): a case report Yusuke Ohara, Tatsuya Oda*, Tsuyoshi Enomoto, Katsuji Hisakura, Yoshimasa Akashi, Koichi Ogawa, Yohei Owada, Yu Domoto, Yoshihiro Miyazaki, Osamu Shimomura, Masanao Kurata and Nobuhiro Ohkohchi Abstract Background: Pancreatic acinar cell carcinoma (PACC), a rare variant of pancreatic malignancy, is generally managed the same way as pancreatic ductal adenocarcinoma (PDAC). -

Laparoscopic Hand-Assisted Total Pancreatectomy: Single Institution Experience of Seven Patients
JOP. J Pancreas (Online) 2020 June 30; 21(3): 57-62. ORIGINAL ARTICLE Laparoscopic Hand-assisted Total Pancreatectomy: Single Institution Experience of Seven Patients Sujit Kulkarni1, Kaylene Barrera2, Rick Selby1, Dilipkumar Parekh1 1Department of Surgery, Keck Medical Center, University of Southern California, Los Angeles, United States 2SUNY Downstate Medical Center, New York City, New York, United States ABSTRACT Background In the past two decades, total pancreatectomy has been associated with improved postoperative and long-term outcomes due to the improvements in surgical technique, better enzyme preparations and diabetes control. While minimally invasive Whipple operation has enjoyed the attention in recent years, the safety and feasibility of a minimally invasive total pancreatectomy is still not established. Methods A retrospective review of minimally invasive total pancreatic resections. Results Seven patients underwent laparoscopic hand- assisted total pancreatectomy between 2005 and 2011. The mean patient age was 58.1 years (58.1 ± 6.45) and the median American Society of Anesthesiologist score was 3. Three patients had diffuse IPMN, two had multiple neuroendocrine tumors and two patients had large cystic lesions in head, body and tail of pancreas. Median operative time was 431 minutes (range 348-590) with 300 cc (range the mortality was 0. Conclusion The laparoscopic hand-assisted total pancreatectomy appears to be a safe and feasible procedure. It is a150-1200) technically of demanding blood loss. procedureThe 90 days requiring postoperative expertise complication in both open rate and of advancedgrade 2 or laparoscopic higher Clavien-Dindo pancreatic classification procedures andwas additional 14% and multi-institutional studies are necessary to further evaluate its role. -

The Evaluation of the Early and Late Postoperative Pancreatic Function and Nutritional Status: Central Pancreatectomy Versus Distal Pancreatectomy
ISSN: 2378-3397 Izumo et al. Int J Surg Res Pract 2017, 4:057 DOI: 10.23937/2378-3397/1410057 Volume 4 | Issue 3 International Journal of Open Access Surgery Research and Practice RESEARCH ARTICLE The Evaluation of the Early and Late Postoperative Pancreatic Func- tion and Nutritional Status: Central Pancreatectomy Versus Distal Pancreatectomy Wataru Izumo*, Ryota Higuchi and Masakazu Yamamoto Department of Surgery, Institute of Gastroenterology, Tokyo Women’s Medical University, Japan *Corresponding author: Wataru Izumo, Department of Surgery, Institute of Gastroenterology, Tokyo Women’s Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo 162-8666, Japan, E-mail: [email protected]; [email protected] Abstract Introduction Background: Central pancreatectomy is performed to The pancreas has both exocrine and endocrine func- preserve pancreatic function in selected patients with low- tions, and plays an important role in digestion and ab- grade tumors. We evaluated short-term and long-term pan- sorption by secreting pancreatic digestive enzymes in creatic function and nutritional status after central or distal pancreatectomy. the pancreatic juice and releasing insulin into the blood. In recent years, central pancreatectomy has been per- Methods: The subjects were 24 patients undergoing central pancreatectomy and 91 patients receiving distal pancrea- formed to preserve pancreatic function in selected pa- tectomy. We retrospectively evaluated body weight, serum tients with low-grade pancreatic tumors, but its short- albumin, hemoglobin A1c, and complications. term and long-term efficacy for achieving this objective Results: After central pancreatectomy, body weight and he- has been not so clear. moglobin A1c did not change significantly up to 60 months postoperatively compared with before surgery, while serum Theoretically, pancreatic exocrine function should albumin was significantly increased at all postoperative as- be better after central pancreatectomy than after other sessments (6, 12, 36, and 60 months, all P < 0.05).