Ex Vivo Resection and Autotransplantation for Pancreatic Neoplasms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gastrostomy Feeding Tubes
Gastrostomy feeding tubes With Dr Anastasia Volovets, Gastroenterologist and Hepatologist, Royal Prince Alfred Hospital, Sydney, Australia Introduction In patients with prolonged inadequate or absent oral intake gastrostomy tubes can be used to provide a route for enteral feeding, hydration, and medication administration. Case 1 - You are a junior doctor on the wards and you’re called to see a 65 year old male, who is day 5 post- stroke with an impaired swallow he is unable to tolerate oral feed and his family is worried he will starve to death. 1. Management of this patient IV fluids do not provide the caloric support or nutrients needed by patients, after 48 hours of impaired oral feeding, enteral feeding should be considered. • Short term this would be a nasogastric tube • Longer term (greater than 6 weeks) a gastrostomy or jejunostomy should be considered 2. Indications for enteral feeding • Neurological disorders causing impaired swallowing and aspiration of food o Stroke (most common) o Traumatic brain injury o Parkinson’s disease • Structural problems o Malignancy obstructing the gastrointestinal tract, this can include upper GI, head, nose or throat. Gastrostomy insertion can be done prophylactically prior to treatment that will impair the functioning or path of the tract such as surgery or radiotherapy 3. Contraindications to gastric feeding tubes • Absolute o High bleeding risk - uncorrected coagulopathy, thrombocytopenia o Chronic liver disease - varies and ascites o Peritonitis or abdominal perforation o Cellulitis at selected -

OT Resource for K9 Overview of Surgical Procedures
OT Resource for K9 Overview of surgical procedures Prepared by: Hannah Woolley Stage Level 1 2 Gynecology/Oncology Surgeries Lymphadenectomy (lymph node dissection) Surgical removal of lymph nodes Radical: most/all of the lymph nodes in tumour area are removed Regional: some of the lymph nodes in the tumour area are removed Omentectomy Surgical procedure to remove the omentum (thin abdominal tissue that encases the stomach, large intestine and other abdominal organs) Indications for omenectomy: Ovarian cancer Sometimes performed in combination with TAH/BSO Posterior Pelvic Exenteration Surgical removal of rectum, anus, portion of the large intestine, ovaries, fallopian tubes and uterus (partial or total removal of the vagina may also be indicated) Indications for pelvic exenteration Gastrointestinal cancer (bowel, colon, rectal) Gynecological cancer (cervical, vaginal, ovarian, vulvar) Radical Cystectomy Surgical removal of the whole bladder and proximal lymph nodes In men, prostate gland is also removed In women, ovaries and uterus may also be removed Following surgery: Urostomy (directs urine through a stoma on the abdomen) Recto sigmoid pouch/Mainz II pouch (segment of the rectum and sigmoid colon used to provide anal urinary diversion) 3 Radical Vulvectomy Surgical removal of entire vulva (labia, clitoris, vestibule, introitus, urethral meatus, glands/ducts) and surrounding lymph nodes Indication for radical vulvectomy Treatment of vulvar cancer (most common) Sentinel Lymph Node Dissection (SLND) Exploratory procedure where the sentinel lymph node is removed and examined to determine if there is lymph node involvement in patients diagnosed with cancer (commonly breast cancer) Total abdominal hysterectomy/bilateral saplingo-oophorectomy (TAH/BSO) Surgical removal of the uterus (including cervix), both fallopian tubes and ovaries Indications for TAH/BSO: Uterine fibroids: benign growths in the muscle of the uterus Endometriosis: condition where uterine tissue grows on structures outside the uterus (i.e. -

Complication Analysis of Distal Pancreatectomy Based on Early Personal Experience
Korean J Hepatobiliary Pancreat Surg 2011;15:243-247 Original Article Complication analysis of distal pancreatectomy based on early personal experience Sung-Jin Park, Hyung-Il Seo, Soo-Hee Go, Sung-Pil Yun, and Ji-Yeon Lee Department of Surgery, Postgraduate School of Medicine, Pusan National University, Busan, Korea Backgrounds/Aims: The objective of this study was to evaluate the relationship between initial personal experiences with distal pancreatectomy and perioperative risk factors, outcomes, and management of pancreatic fistulas. Methods: Between May, 2007 and May, 2010, a total of 28 patients who had undergone elective distal pancreatectomy were evaluated for this study. Perioperative factors and the occurrence of pancreatic fistula were analyzed on the basis of International Study Group of Pancreatic Fistula (ISGPF) criteria. Results: There were sixteen cases of benign neo- plasms and twelve cases of malignant tumors. The remnant pancreas was manually sutured with ligation of the pancre- atic duct (n=14), auto-suture stapling along with manual sutures (n=12), or stapling alone (n=2). According to the ISGPF classification, morbidity and mortality associated with pancreatic fistulas was 42.9% (n=12) and 0%, respectively. These pancreatic fistulae were classified as grade A in 8 cases (28.6%), grade B in 3 cases (10.7%), and grade C in one case (3.6%). All patients with pancreatic fistula were treated conservatively. Conclusions: Perioperative factors do not affect the risk of pancreatic fistula. Adequate drainage is the most effective method for management of a pancreatic fistula after distal pancreatectomy. (Korean J Hepatobiliary Pancreat Surg 2011;15:243-247) Key Words: Distal pancreatectomy; Pancreatic fistula; Complication; Leak INTRODUCTION Study Group for Pancreatic Fistula (ISGPF). -

Pancreaticogastrostomy
eCommons@AKU Section of General Surgery Department of Surgery October 2017 Pancreaticogastrostomy - an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy - experience from a tertiary care center of Pakistan Tabish Chawla Aga Khan University, [email protected] Hassaan Bari Aga Khan University Shahrukh Effendi Follow this and additional works at: https://ecommons.aku.edu/pakistan_fhs_mc_surg_gen Part of the Surgery Commons Recommended Citation Chawla, T., Bari, H., Effendi, S. (2017). Pancreaticogastrostomy - an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy - experience from a tertiary care center of Pakistan. Journal of Pakistan Medical Association, 67(10), 1621-1624. Available at: https://ecommons.aku.edu/pakistan_fhs_mc_surg_gen/76 1621 CASE SERIES Pancreaticogastrostomy — an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy — experience from a tertiary care center of Pakistan Tabish Chawla, Hassaan Bari, Shahrukh Effendi Abstract as part of PD. Therefore it was associated with high Whipple's pancreaticoduodenectomy has been refined morbidity and mortality resulting from high rates of over the years to be a safe operation though the leakage from pancreatic stump. morbidity rate still remains high (30-50%). Pancreatic Pancreatcogastrostomy is a repopularized technique fistula is the most important cause of mortality which has been described previously in literature. 3 This following pancreaticoduodenectomy. To prevent it, study was done to review the experience of PG being surgeons have used two anastomotic techniques: done as an alternate to PJ after PD. pancreaticojejunostomy and pancreaticogastrostomy. Recent studies found that pancreaticogastrostomy is Material and Methods associated with fewer overall complications than It is a case series collected at the Department of Surgery of pancreaticojejunostomy. -

High Risk Percutaneous Endoscopic Gastrostomy Tubes: Issues to Consider
NUTRITIONINFLAMMATORY ISSUES BOWEL IN GASTROENTEROLOGY, DISEASE: A PRACTICAL SERIES APPROACH, #105 SERIES #73 Carol Rees Parrish, M.S., R.D., Series Editor High Risk Percutaneous Endoscopic Gastrostomy Tubes: Issues to Consider Iris Vance Neeral Shah Percutaneous endoscopy gastrostomy (PEG) tubes are a valuable tool for providing long- term enteral nutrition or gastric decompression; certain circumstances that complicate PEG placement warrant novel approaches and merit review and discussion. Ascites and portal hypertension with varices have been associated with poorer outcomes. Bleeding is one of the most common serious complications affecting approximately 2.5% of all procedures. This article will review what evidence exists in these high risk scenarios and attempt to provide more clarity when considering these challenging clinical circumstances. INTRODUCTION ince the first Percutaneous Endoscopic has been found by multiple authors to portend a poor Gastrostomy tube was placed in 1979 (1), they prognosis in PEG placement (3,4, 5,6,7,8). This review Shave become an invaluable tool for providing will endeavor to provide more clarity when considering long-term enteral nutrition (EN) and are commonly used these challenging clinical circumstances. in patients with dysphagia following stroke, disabling motor neuron diseases such as multiple sclerosis and Ascites & Gastric Varices amyotrophic lateral sclerosis, and in those with head The presence of ascites is frequently viewed as a and neck cancer.They are also used for patients with relative, if not absolute, contraindication to PEG prolonged mechanical intubation, as well as gastric placement. Ascites adds technical difficulties and the decompression in those with severe gastroparesis, risk for potential complications (see Table 1). -
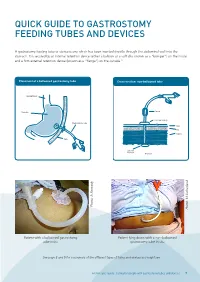
Quick Guide to Gastrostomy Feeding Tubes and Devices
QUICK GUIDE TO GASTROSTOMY FEEDING TUBES AND DEVICES A gastrostomy feeding tube or device is one which has been inserted directly through the abdominal wall into the stomach. It is secured by an internal retention device (either a balloon or a soft disc known as a “bumper”) on the inside and a firm external retention device (known as a “flange”) on the outside.11 Placement of a ballooned gastrostomy tube Cross-section: non-ballooned tube Oesophagus Stomach Clamp External Flange Gastrostomy tube Skin Fat Muscle Skin Internal Bumper Stomach Photo: APhoto: Kennedy Photo: MPhoto: Sutherland Patient with a ballooned gastrostomy Patient lying down with a non-ballooned tube insitu gastrostomy tube in situ See page 8 and 9 for a summary of the different types of tubes and devices you might see. A Clinician’s Guide: Caring for people with gastrostomy tubes and devices 7 Common features of gastrostomy feeding tubes and devices include, but are not limited to: Refer to manufacturer’s guidelines for advice on brand specific tube and device features Ballooned Gastrostomy Tube Ballooned Gastrostomy Tube With side port Without side port Feeding Port Feeding Port (Enteral Dispenser (Enteral Dispenser and Feed Bag and Feed Bag connect here) connect here) ml/cc Balloon Port Balloon Port ml/cc Side Port (X ml/cc) (X ml/cc) French (size) [For example:16/18/20] French (size) [For example:16/18/20] FR FR cm markings cm markings External External Flange Flange Balloon Balloon Non-ballooned Gastrostomy Tube Non-ballooned Gastrostomy Tube with collapsible internal -

Chemical Pancreatectomy Treats Chronic Pancreatitis While Preserving Endocrine Function in Preclinical Models
Chemical pancreatectomy treats chronic pancreatitis while preserving endocrine function in preclinical models Mohamed Saleh, … , Krishna Prasadan, George Gittes J Clin Invest. 2020. https://doi.org/10.1172/JCI143301. Research In-Press Preview Endocrinology Gastroenterology Chronic pancreatitis affects over 250,000 people in the US and millions worldwide. It is associated with chronic debilitating pain, pancreatic exocrine failure, high-risk of pancreatic cancer, and usually progresses to diabetes. Treatment options are limited and ineffective. We developed a new potential therapy, wherein a pancreatic ductal infusion of 1-2% acetic acid in mice and non-human primates resulted in a non-regenerative, near-complete ablation of the exocrine pancreas, with complete preservation of the islets. Pancreatic ductal infusion of acetic acid in a mouse model of chronic pancreatitis led to resolution of chronic inflammation and pancreatitis-associated pain. Furthermore, acetic acid-treated animals showed improved glucose tolerance and insulin secretion. The loss of exocrine tissue in this procedure would not typically require further management in patients with chronic pancreatitis because they usually have pancreatic exocrine failure requiring dietary enzyme supplements. Thus, this procedure, which should be readily translatable to humans through an endoscopic retrograde cholangiopancreatography (ERCP), may offer a potential innovative non-surgical therapy for chronic pancreatitis that relieves pain and prevents the progression of pancreatic diabetes. Find the latest version: https://jci.me/143301/pdf Chemical pancreatectomy treats chronic pancreatitis while preserving endocrine function in preclinical models Authors: Mohamed Saleh1,2, *Kartikeya Sharma1, *Ranjeet Kalsi1, Joseph Fusco1, Anuradha Sehrawat1, Jami L. Saloman3, Ping Guo4, Ting Zhang 1, Nada Mohamed1, Yan Wang1, Krishna Prasadan1, George K. -

Original Article
ABCD Arq Bras Cir Dig Original Article - Technique 2018;31(3):e1395 DOI: /10.1590/0102-672020180001e1395 LAPAROSCOPIC DISTAL PANCREATECTOMY WITH SPLEEN PRESERVATION Pancreatectomia distal laparoscópica com preservação esplênica Sergio Renato PAIS-COSTA1,2, Guilherme Costa Crispim de SOUSA1,2, Sergio Luiz Melo ARAUJO1,2, Olímpia Alves Teixeira LIMA1,2 How to cite this article: Pais-Costa SR, Sousa GCC, Araujo SLM, Lima OAT. Laparoscopic distal pancreatectomy with preservation of the spleen. ABCD Arq Bras Cir Dig. 2018;31(3):e1395. DOI: /10.1590/0102-672020180001e1395 From the 1Hospital Santa Lúcia, Brasília, DF ABSTRACT - Background: Laparoscopic distal pancreatectomy has been the choice for resection and 2Hospital Brasília, Brasília, DF, Brasil. of distal pancreas lesions due many advantages over open approach. Spleen preservation technique seems minimizes infectious complications in long-term outcome. Aim: To present the results of laparoscopic distal pancreatectomies with spleen preservation by Kimura´s technique (preservation of spleen blood vessels) performed by single surgical team. Methods: Retrospective case series aiming to evaluate both short and long-term outcomes of laparoscopic distal pancreatectomies with spleen preservation. Results: A total of 54 laparoscopic distal pancreatectomies were performed, in which 26 were laparoscopic distal pancreatectomies with spleen preservation by Kimura´s technique. Mean age was 47.9 years-old (21-75) where 61.5% were female. Mean BMI was 28.5 kg/m² (18-38.8). Mean diameter of lesion was 4.3 cm (1.8- 7.5). Mean operative time was 144.1 min (90-200). Intraoperative bleeding was 119.2 ml (50- 600). Conversion to laparotomy 3% (n=1). -
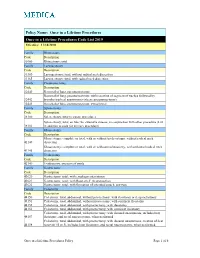
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Postoperative Biological and Physiological Gastrointestinal Changes After Whipple Procedure
Ju ry [ rnal e ul rg d u e S C f h o i l r u a Journal of Surgery r n g r i u e o ] J ISSN: 1584-9341 [Jurnalul de Chirurgie] Review Article Open Access Postoperative Biological and Physiological Gastrointestinal Changes after Whipple Procedure Daniel Timofte*, Ionescu Lidia and Lăcrămioara Ochiuz 3rd Surgical Unit, St. Spiridon Hospital, Bd. Independenței, No 1700111, Iasi, Romania Abstract In the last years there was an increased interest towards the pancreatic cancer, especially considering its growing incidence (rapidly becoming the fifth cause of death by cancer in the developed countries), lack of any sustainable markers and/or risk factors and the chilling fact that almost 95% of the patients with this disorder are presenting to the hospital in the advanced and unresectable stages. Even more, although known and developed for almost 70 years, the surgical approach for the pancreatic cancer is a subject of debate because its efficacy and postoperative biological changes. It is known that the most common surgery in chronic pancreatitis and pancreatic cancer is represented by the Whipple pancreatico duodenectomy. Still, after an extended resection and reconstruction of the upper gastrointestinal tract, it seems that the digestive physiology can be disrupted. In this way, in the present mini-review we will describe some postoperative gastrointestinal biological and physiological changes after Whipple procedure, by mainly focusing on the gastrointestinal motility, bone demineralization, dumping and re-resection, as well as on the affected pancreatic function, postoperative weight loss and remnant pancreatic fibrosis and how the management of this related pathological aspects can be applied in these cases. -

Colo-Pancreaticoduodenectomy for Locally Advanced Colon Carcinoma- Feasibility in Patients Manifesting As Acute Abdomen
Colo-pancreaticoduodenectomy for Locally Advanced Colon Carcinoma- feasibility in patients manifesting as Acute Abdomen Joe-Bin Chen Taichung VGH: Taichung Veterans General Hospital Shao-Ciao Luo Taichung VGH: Taichung Veterans General Hospital Chou-Chen Chen Taichung Veterans General Hospital Cheng chung Wu ( [email protected] ) Taichung Veterans General Hospital Yun Yen Taichung Veterans General Hospital Chuan-Hsun Chang Taichung Veterans General Hospital Yun-An Chen Taichung Veterans General Hospital Fang-Ku P’eng Taichung Veterans General Hospital Research article Keywords: locally advanced colon carcinoma, pancreaticoduodenectomy, colectomy, acute abdomen Posted Date: January 27th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-102628/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on February 27th, 2021. See the published version at https://doi.org/10.1186/s13017-021-00351-6. Page 1/12 Abstract Background For locally advanced colon carcinoma that invades duodenum and/or pancreatic head is en-bloc right hemicolectomy plus pancreaticoduodenectomy (PD). This procedure may be also named as colo-pancreaticoduodenectomy (cPD). Patients with such carcinoma may abdomen. Emergent PD often leads to high postoperative morbidity and mortality. Here, we aimed to evaluate the feasibility and outcomes of emergent cPD, for patients with advanced colon carcinoma, manifest acute abdomen condition. Patients and Methods We retrospectively reviewed of 4,793 patients of colorectal cancer, receiving curative colectomy, during the period from 1993 and 2017. Among them, 30 had locally advanced right colon cancer and had received cPD. Among them, surgery of 11 patients was performed in emergent conditions (bowel obstruction 6, perforation 3, tumor bleeding 2). -

Gastroparesis and Dumping Syndrome: Current Concepts and Management
Journal of Clinical Medicine Review Gastroparesis and Dumping Syndrome: Current Concepts and Management Stephan R. Vavricka 1,2,* and Thomas Greuter 2 1 Center of Gastroenterology and Hepatology, CH-8048 Zurich, Switzerland 2 Department of Gastroenterology and Hepatology, University Hospital Zurich, CH-8091 Zurich, Switzerland * Correspondence: [email protected] Received: 21 June 2019; Accepted: 23 July 2019; Published: 29 July 2019 Abstract: Gastroparesis and dumping syndrome both evolve from a disturbed gastric emptying mechanism. Although gastroparesis results from delayed gastric emptying and dumping syndrome from accelerated emptying of the stomach, the two entities share several similarities among which are an underestimated prevalence, considerable impairment of quality of life, the need for a multidisciplinary team setting, and a step-up treatment approach. In the following review, we will present an overview of the most important clinical aspects of gastroparesis and dumping syndrome including epidemiology, pathophysiology, presentation, and diagnostics. Finally, we highlight promising therapeutic options that might be available in the future. Keywords: gastroparesis; dumping syndrome; pathophysiology; clinical presentation; treatment 1. Introduction Gastroparesis and dumping syndrome both evolve from a disturbed gastric emptying mechanism. While gastroparesis results from significantly delayed gastric emptying, dumping syndrome is a consequence of increased flux of food into the small bowel [1,2]. The two entities share several important similarities: (i) gastroparesis and dumping syndrome are frequent, but also frequently overlooked; (ii) they affect patient’s quality of life considerably due to possibly debilitating symptoms; (iii) patients should be taken care of within a multidisciplinary team setting; and (iv) treatment should follow a step-up approach from dietary modifications and patient education to pharmacological interventions and, finally, surgical procedures and/or enteral feeding.