Pancreatic Exocrine Insufficiency and Enteral Feeding: a Practical Guide with Case Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OT Resource for K9 Overview of Surgical Procedures
OT Resource for K9 Overview of surgical procedures Prepared by: Hannah Woolley Stage Level 1 2 Gynecology/Oncology Surgeries Lymphadenectomy (lymph node dissection) Surgical removal of lymph nodes Radical: most/all of the lymph nodes in tumour area are removed Regional: some of the lymph nodes in the tumour area are removed Omentectomy Surgical procedure to remove the omentum (thin abdominal tissue that encases the stomach, large intestine and other abdominal organs) Indications for omenectomy: Ovarian cancer Sometimes performed in combination with TAH/BSO Posterior Pelvic Exenteration Surgical removal of rectum, anus, portion of the large intestine, ovaries, fallopian tubes and uterus (partial or total removal of the vagina may also be indicated) Indications for pelvic exenteration Gastrointestinal cancer (bowel, colon, rectal) Gynecological cancer (cervical, vaginal, ovarian, vulvar) Radical Cystectomy Surgical removal of the whole bladder and proximal lymph nodes In men, prostate gland is also removed In women, ovaries and uterus may also be removed Following surgery: Urostomy (directs urine through a stoma on the abdomen) Recto sigmoid pouch/Mainz II pouch (segment of the rectum and sigmoid colon used to provide anal urinary diversion) 3 Radical Vulvectomy Surgical removal of entire vulva (labia, clitoris, vestibule, introitus, urethral meatus, glands/ducts) and surrounding lymph nodes Indication for radical vulvectomy Treatment of vulvar cancer (most common) Sentinel Lymph Node Dissection (SLND) Exploratory procedure where the sentinel lymph node is removed and examined to determine if there is lymph node involvement in patients diagnosed with cancer (commonly breast cancer) Total abdominal hysterectomy/bilateral saplingo-oophorectomy (TAH/BSO) Surgical removal of the uterus (including cervix), both fallopian tubes and ovaries Indications for TAH/BSO: Uterine fibroids: benign growths in the muscle of the uterus Endometriosis: condition where uterine tissue grows on structures outside the uterus (i.e. -

Different Types of Pancreatico-Enteric Anastomosis
Review Article Different types of pancreatico-enteric anastomosis Savio George Barreto1,2, Parul J. Shukla3 1Hepatobiliary and Oesophagogastric Unit, Division of Surgery and Perioperative Medicine, Flinders Medical Centre, Adelaide, Australia; 2College of Medicine and Public Health, Flinders University, Bedford Park SA, Australia; 3Department of Surgery, Weill Cornell Medical College & New York Presbyterian Hospital, New York, USA Contributions: (I) Conception and design: SG Barreto, PJ Shukla; (II) Administrative support: None; (III) Provision of study materials or patients: SG Barreto; (IV) Collection and assembly of data: SG Barreto; (V) Data analysis and interpretation: SG Barreto; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Parul J. Shukla. Department of Surgery, Weill Cornell Medical College & New York Presbyterian Hospital, New York, USA. Email: [email protected]. Abstract: The pancreatico-enteric anastomosis has widely been regarded as the ‘Achilles heel’ of the modern day, single-stage, pancreatoduodenectomy (PD). A review of the literature was carried out to address the evolution of the pancreatico-enteric anastomosis following PD, the spectrum of anastomoses performed around the world, and finally present the current evidence in support of each anastomosis. Pancreaticogastrostomy (PG) and pancreaticojejunostomy (PJ) are the most common forms of pancreatico- enteric reconstruction following PD. There is no difference in postoperative pancreatic fistula (POPF) rates between PG and PJ, as well as individual variations, except in a high-risk anastomosis where performance of a PJ may be preferred. The routine use of glue, trans-anastomotic stents or omental wrapping is of no proven benefit. Externalised trans-anastomotic stents may have a role in mitigating the risk of a clinically relevant POPF in high-risk anastomoses. -

Complication Analysis of Distal Pancreatectomy Based on Early Personal Experience
Korean J Hepatobiliary Pancreat Surg 2011;15:243-247 Original Article Complication analysis of distal pancreatectomy based on early personal experience Sung-Jin Park, Hyung-Il Seo, Soo-Hee Go, Sung-Pil Yun, and Ji-Yeon Lee Department of Surgery, Postgraduate School of Medicine, Pusan National University, Busan, Korea Backgrounds/Aims: The objective of this study was to evaluate the relationship between initial personal experiences with distal pancreatectomy and perioperative risk factors, outcomes, and management of pancreatic fistulas. Methods: Between May, 2007 and May, 2010, a total of 28 patients who had undergone elective distal pancreatectomy were evaluated for this study. Perioperative factors and the occurrence of pancreatic fistula were analyzed on the basis of International Study Group of Pancreatic Fistula (ISGPF) criteria. Results: There were sixteen cases of benign neo- plasms and twelve cases of malignant tumors. The remnant pancreas was manually sutured with ligation of the pancre- atic duct (n=14), auto-suture stapling along with manual sutures (n=12), or stapling alone (n=2). According to the ISGPF classification, morbidity and mortality associated with pancreatic fistulas was 42.9% (n=12) and 0%, respectively. These pancreatic fistulae were classified as grade A in 8 cases (28.6%), grade B in 3 cases (10.7%), and grade C in one case (3.6%). All patients with pancreatic fistula were treated conservatively. Conclusions: Perioperative factors do not affect the risk of pancreatic fistula. Adequate drainage is the most effective method for management of a pancreatic fistula after distal pancreatectomy. (Korean J Hepatobiliary Pancreat Surg 2011;15:243-247) Key Words: Distal pancreatectomy; Pancreatic fistula; Complication; Leak INTRODUCTION Study Group for Pancreatic Fistula (ISGPF). -

Chemical Pancreatectomy Treats Chronic Pancreatitis While Preserving Endocrine Function in Preclinical Models
Chemical pancreatectomy treats chronic pancreatitis while preserving endocrine function in preclinical models Mohamed Saleh, … , Krishna Prasadan, George Gittes J Clin Invest. 2020. https://doi.org/10.1172/JCI143301. Research In-Press Preview Endocrinology Gastroenterology Chronic pancreatitis affects over 250,000 people in the US and millions worldwide. It is associated with chronic debilitating pain, pancreatic exocrine failure, high-risk of pancreatic cancer, and usually progresses to diabetes. Treatment options are limited and ineffective. We developed a new potential therapy, wherein a pancreatic ductal infusion of 1-2% acetic acid in mice and non-human primates resulted in a non-regenerative, near-complete ablation of the exocrine pancreas, with complete preservation of the islets. Pancreatic ductal infusion of acetic acid in a mouse model of chronic pancreatitis led to resolution of chronic inflammation and pancreatitis-associated pain. Furthermore, acetic acid-treated animals showed improved glucose tolerance and insulin secretion. The loss of exocrine tissue in this procedure would not typically require further management in patients with chronic pancreatitis because they usually have pancreatic exocrine failure requiring dietary enzyme supplements. Thus, this procedure, which should be readily translatable to humans through an endoscopic retrograde cholangiopancreatography (ERCP), may offer a potential innovative non-surgical therapy for chronic pancreatitis that relieves pain and prevents the progression of pancreatic diabetes. Find the latest version: https://jci.me/143301/pdf Chemical pancreatectomy treats chronic pancreatitis while preserving endocrine function in preclinical models Authors: Mohamed Saleh1,2, *Kartikeya Sharma1, *Ranjeet Kalsi1, Joseph Fusco1, Anuradha Sehrawat1, Jami L. Saloman3, Ping Guo4, Ting Zhang 1, Nada Mohamed1, Yan Wang1, Krishna Prasadan1, George K. -

Diapause in the Mosquito Culex Pipiens Evokes a Metabolic Switch from Blood Feeding to Sugar Gluttony
Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony Rebecca M. Robich* and David L. Denlinger† Department of Entomology, Ohio State University, 318 West 12th Avenue, Columbus, OH 43210 Contributed by David L. Denlinger, September 12, 2005 A key characteristic of overwintering dormancy (diapause) in the Although the physiological and ecological aspects of blood and mosquito Culex pipiens is the switch in females from blood feeding sugar feeding in diapausing C. pipiens have been well described, the to sugar gluttony. We present evidence demonstrating that genes molecular events that contribute to this metabolic decision have not encoding enzymes needed to digest a blood meal (trypsin and a been explored. In this study, we used suppressive subtractive chymotrypsin-like protease) are down-regulated in diapause-des- hybridization (SSH) to isolate three clones linked to this metabolic tined females, and that concurrently, a gene associated with the decision: fatty acid synthase, trypsin, and chymotrypsin-like serine accumulation of lipid reserves (fatty acid synthase) is highly up- protease. We then used these clones to probe the metabolic path- regulated. As the females then enter diapause, fatty acid synthase ways associated with the mosquito’s metabolic decision to enter and is only sporadically expressed, and expression of trypsin and terminate diapause. Because both short day length and low tem- chymotrypsin-like remains undetectable. Late in diapause (2–3 perature program diapause in C. pipiens, we also distinguished months at 18°C), the genes encoding the digestive enzymes begin between temperature and photoperiodic effects. We concluded to be expressed as the female prepares to take a blood meal upon that the short-day programming of diapause results in the down- the termination of diapause. -

Wsn 40 (2016) 147-162 Eissn 2392-2192
Available online at www.worldscientificnews.com WSN 40 (2016) 147-162 EISSN 2392-2192 Utilization of mulberry leaves treated with seed powder cowpea, Vigna unguiculata (L) for feeding the fifth instar larvae of silkworm, Bombyx mori (L) (Race: PM x CSR2) Vitthalrao B. Khyade1,*, Atharv Atul Gosavi2 1Department of Zoology, Shardabai Pawar Mahila Mahavidyalaya, Shardanagar Tal. Baramati; Dist. Pune - 413115, India 2Agriculture Development Trust Agri Polytechnic, Sharadanagar, Malegaon Colony, Tal: Baramati, Dist: Pune. PIN: 413115 Maharashtra, India *E-mail address: [email protected] ABSTRACT The present attempt was to screen the changes in the cocoon parameters; silk filament parameters and activities of biochemical reactions catalyzed by the midgut enzymes fifth instsr larvae of silkworm fed with mulberry leaves treated with aqueous solution of seed powder of Cowpeas (Vigna unguiculata). The cowpea seed powder was dissolved in distilled water and diluted to 2.5%, 5%, 7.5%, and 10% concentrations. Fresh mulberry leaves were dipped in each concentration of aqueous solution of cowpea seed powder for half an hour. 1000 ml solution was used for 100 grams of mulberry leaves. Treated mulberry leaves were drained off completely and then used for feeding. The mulberry leaves were fed five times per day at the rate of 100 grams per 100 larvae for each time. Untreated group of larvae were feed with untreated mulberry leaves. Water treated group of larvae were feed with water treated mulberry leaves. The experimental groups of larvae were feed with feed separately with 2.5 percent cowpea treated; 5.00 percent cowpea treated; 7.5 percent cowpea treated and 10.00 percent cowpea treated mulberry leaves. -

Original Article
ABCD Arq Bras Cir Dig Original Article - Technique 2018;31(3):e1395 DOI: /10.1590/0102-672020180001e1395 LAPAROSCOPIC DISTAL PANCREATECTOMY WITH SPLEEN PRESERVATION Pancreatectomia distal laparoscópica com preservação esplênica Sergio Renato PAIS-COSTA1,2, Guilherme Costa Crispim de SOUSA1,2, Sergio Luiz Melo ARAUJO1,2, Olímpia Alves Teixeira LIMA1,2 How to cite this article: Pais-Costa SR, Sousa GCC, Araujo SLM, Lima OAT. Laparoscopic distal pancreatectomy with preservation of the spleen. ABCD Arq Bras Cir Dig. 2018;31(3):e1395. DOI: /10.1590/0102-672020180001e1395 From the 1Hospital Santa Lúcia, Brasília, DF ABSTRACT - Background: Laparoscopic distal pancreatectomy has been the choice for resection and 2Hospital Brasília, Brasília, DF, Brasil. of distal pancreas lesions due many advantages over open approach. Spleen preservation technique seems minimizes infectious complications in long-term outcome. Aim: To present the results of laparoscopic distal pancreatectomies with spleen preservation by Kimura´s technique (preservation of spleen blood vessels) performed by single surgical team. Methods: Retrospective case series aiming to evaluate both short and long-term outcomes of laparoscopic distal pancreatectomies with spleen preservation. Results: A total of 54 laparoscopic distal pancreatectomies were performed, in which 26 were laparoscopic distal pancreatectomies with spleen preservation by Kimura´s technique. Mean age was 47.9 years-old (21-75) where 61.5% were female. Mean BMI was 28.5 kg/m² (18-38.8). Mean diameter of lesion was 4.3 cm (1.8- 7.5). Mean operative time was 144.1 min (90-200). Intraoperative bleeding was 119.2 ml (50- 600). Conversion to laparotomy 3% (n=1). -

Digestive Enzymes: the Key to Optimum Health
Education Article Digestive Enzymes: The Key to Optimum Health Digestion is essential for good health. Unlocking nutrients from foods is a complex process. In order to break down food we rely on optimal levels and function of a special set of proteins called digestive enzymes. These proteins are found in the saliva and also in the small intestines. Without the combined actions of our digestive enzymes we would simply be unable to absorb many nutrients that we need to maintain good health. This is why reduced levels of digestive enzymes can be linked to a wide-range of symptoms within the gut and beyond. Digestive enzymes, along with stomach acid, play a crucial role in the initial stages of digestion, i.e. the breaking down of the food that we eat. The main classes of human digestive enzymes include proteases, lipases and carbohydrases, which respectively break down the macronutrients protein, fats and carbohydrates. If we do not efficiently digest these foods then vital nutrients such as essential fats, vitamins, minerals and phytonutrients cannot be absorbed. What is more, undigested or partially digested food passes through into the large intestines and is fermented by the resident colonic bacteria, causing unpleasant symptoms such as bloating and flatulence and contributing to a toxic bowel. Naturopaths believe toxicity within the bowel is the root of all disease. We do not make digestive enzymes for every type of food that we eat, such as gluten and phytic acid found in some grains and cereals and lactose, a sugar found in milk. This means our bodies may find it difficult to digest these types of foods. -
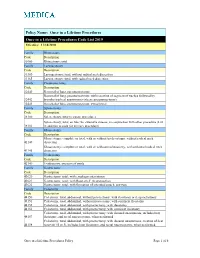
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Leapfrog Hospital Survey Hard Copy
Leapfrog Hospital Survey Hard Copy QUESTIONS & REPORTING PERIODS ENDNOTES MEASURE SPECIFICATIONS FAQS Table of Contents Welcome to the 2016 Leapfrog Hospital Survey........................................................................................... 6 Important Notes about the 2016 Survey ............................................................................................ 6 Overview of the 2016 Leapfrog Hospital Survey ................................................................................ 7 Pre-Submission Checklist .................................................................................................................. 9 Instructions for Submitting a Leapfrog Hospital Survey ................................................................... 10 Helpful Tips for Verifying Submission ......................................................................................... 11 Tips for updating or correcting a previously submitted Leapfrog Hospital Survey ...................... 11 Deadlines ......................................................................................................................................... 13 Deadlines for the 2016 Leapfrog Hospital Survey ...................................................................... 13 Deadlines Related to the Hospital Safety Score ......................................................................... 13 Technical Assistance....................................................................................................................... -

Incidental Presentation of a Pancreatico-Colonic Fistula Case Report and Literature Review
JOP. J Pancreas (Online) 2019 Mar 29; 20(2):51-56. REVIEW ARTICLE Incidental Presentation of A Pancreatico-Colonic Fistula Case Report and Literature Review Mar Achalandabaso Boira1, Luis Ferreira 2, Caroline Conlon3, Caroline Conlon4 1 St Vincent´s University Hospital, Dublin, Ireland 2 Queen Elisabeth Hospital, Birmingham, United Kingdom 3,4 Department of Surgery, Trinty College Dublin, Dublin ABSTRACT Introduction Pancreatitis can be associated with walled off necrosis and fistula resulting in significant morbidity and mortality. We present a case of a patient without previous history of abdominal pain or acute pancreatitis who, while being investigated with respiratoryMaterial andsymptoms, Methods was diagnosed with lung cancer and was found to have a Pancreatico-colonic fistula. The aim of the study was to perform a systematic review on pancreatico-colonic fistula and assess if conservative management can be possible in specific situations. AvailableResults-Case literature reportin English was reviewed until January 2019. PRISMA guidelines were followed identifying 91 records. After screening, seven papers reporting seven patients were identified as definitive pancreatico-colonic fistula and included. All of these were case-reports. A sixty-seven-year-old man with smoking history and strong alcohol intake presented with weight loss and non-productive cough. There was no prior history of pancreatitis or significant abdominal pain. A chest x-ray, showed a left upper lobe pulmonary lesion. Computed tomography demonstrated an abnormal pancreas with intra-panrenchymal gasLiterature along body review and tail tracking back towards the transverse colon. A gastrografin enema showed pancreatic duct filled with contrast retrogradely through the transverse colon. As he was asymptomatic from the pancreatic standpoint a conservative approach was adopted. -

Partial Characterization of Hepatopancreatic and Extracellular
Aquaculture International Archimer June 2011, Volume 19, Number 3, Pages 445-457 http://archimer.ifremer.fr http://dx.doi.org/10.1007/s10499-010-9360-5 © Springer Science+Business Media B.V. 2010 The original publication is available at http://www.springerlink.com is available on the publisher Web site Web publisher the on available is Partial characterization of hepatopancreatic and extracellular digestive proteinases of wild and cultivated Octopus maya R. Martínez1, R. Sántos2, A. Álvarez3, G. Cuzón4, L. Arena5, M. Mascaró5, C. Pascual5 and C. Rosas5, * 1 División de Posgrado, FMVZ, Universidad Autónoma de Yucatán, Mérida, Yucatán, Mexico 2 authenticated version authenticated Departamento de Nutrición, FMVZ, Universidad Autónoma de Yucatán, Mérida, Yucatán, Mexico - 3 Unidad de Ciencias Biológicas, UJAT, Villahermosa, Tabasco, Mexico 4 Ifremer, Tahiti, French Polynesia 5 Unidad Multidisciplinaria de Docencia e Investigación, Facultad de Ciencias, Universidad Nacional Autónoma de México, Puerto de abrigo s/n, Sisal, Yucatan, Mexico *: Corresponding author : C. Rosas, email address : [email protected] Abstract: Abstract Proteinases from hepatopancreas (HP) and gastric juice (GJ) from wild and cultured red octopus (Octopus maya) were characterized. Hepatopancreas assays revealed optimal activity at pH 4, 9–10 and 10 for wild and pH 3, 8, and 9, for cultured octopuses, for total proteinases, trypsin and chymotrypsin, respectively. In the gastric juice, maximum activity was recorded at pH 6, 8, and 7 for total proteinases, trypsin, and chymotrypsin, respectively for both wild and cultured octopus. A reduction on enzyme activity of 70 and 20% was observed in HP and GJ extracts, respectively when protease inhibitor Pepstatin A was used.