Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Mechanisms Underlying Ketamine-Mediated Inhibition Of
Anesthesiology 2005; 102:93–101 © 2004 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Molecular Mechanisms Underlying Ketamine-mediated Inhibition of Sarcolemmal Adenosine Triphosphate- sensitive Potassium Channels Takashi Kawano, M.D.,* Shuzo Oshita, M.D.,† Akira Takahashi, M.D.,‡ Yasuo Tsutsumi, M.D.,* Katsuya Tanaka, M.D.,§ Yoshinobu Tomiyama, M.D., Hiroshi Kitahata, M.D.,# Yutaka Nakaya, M.D.** Background: Ketamine inhibits adenosine triphosphate-sen- sensitive channel pore.3 These channels, as metabolic sitive potassium (KATP) channels, which results in the blocking sensors, are associated with such cellular functions as of ischemic preconditioning in the heart and inhibition of va- insulin secretion, cardiac preconditioning, vasodilata- sorelaxation induced by KATP channel openers. In the current 4–7 Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/102/1/93/357819/0000542-200501000-00017.pdf by guest on 27 September 2021 study, the authors investigated the molecular mechanisms of tion, and neuroprotection. ketamine’s actions on sarcolemmal KATP channels that are re- In cardiac myocytes, intravenous general anesthetics, associated by expressed subunits, inwardly rectifying potas- such as ketamine racemate, propofol, and thiamylal, di- sium channels (Kir6.1 or Kir6.2) and sulfonylurea receptors 8–10 rectly inhibit native sarcolemmal KATP channels. Al- (SUR1, SUR2A, or SUR2B). though these observations suggest that intravenous an- Methods: The authors used inside-out patch clamp configura- tions to investigate the effects of ketamine on the activities of esthetics may impair the endogenous organ protective reassociated Kir6.0/SUR channels containing wild-type, mutant, mechanisms mediated by KATP channels, the possibility or chimeric SURs expressed in COS-7 cells. -
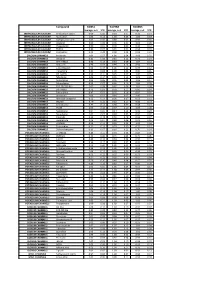
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Label-Free Cell Phenotypic Profiling Decodes the Composition And
OPEN Label-free cell phenotypic profiling SUBJECT AREAS: decodes the composition and signaling POTASSIUM CHANNELS SENSORS AND PROBES of an endogenous ATP-sensitive Received potassium channel 28 January 2014 Haiyan Sun1*, Ying Wei1, Huayun Deng1, Qiaojie Xiong2{, Min Li2, Joydeep Lahiri1 & Ye Fang1 Accepted 24 April 2014 1Biochemical Technologies, Science and Technology Division, Corning Incorporated, Corning, NY 14831, United States of Published America, 2The Solomon H. Snyder Department of Neuroscience and High Throughput Biology Center, Johns Hopkins University 12 May 2014 School of Medicine, Baltimore, Maryland 21205, United States of America. Current technologies for studying ion channels are fundamentally limited because of their inability to Correspondence and functionally link ion channel activity to cellular pathways. Herein, we report the use of label-free cell requests for materials phenotypic profiling to decode the composition and signaling of an endogenous ATP-sensitive potassium should be addressed to ion channel (KATP) in HepG2C3A, a hepatocellular carcinoma cell line. Label-free cell phenotypic agonist Y.F. (fangy2@corning. profiling showed that pinacidil triggered characteristically similar dynamic mass redistribution (DMR) com) signals in A431, A549, HT29 and HepG2C3A, but not in HepG2 cells. Reverse transcriptase PCR, RNAi knockdown, and KATP blocker profiling showed that the pinacidil DMR is due to the activation of SUR2/ Kir6.2 KATP channels in HepG2C3A cells. Kinase inhibition and RNAi knockdown showed that the pinacidil * Current address: activated KATP channels trigger signaling through Rho kinase and Janus kinase-3, and cause actin remodeling. The results are the first demonstration of a label-free methodology to characterize the Biodesign Institute, composition and signaling of an endogenous ATP-sensitive potassium ion channel. -

Thiruttu Tuomittur
THIRUTTUUS009782416B2 TUOMITTUR (12 ) United States Patent ( 10 ) Patent No. : US 9 ,782 ,416 B2 Cowen (45 ) Date of Patent: Oct. 10 , 2017 ( 54 ) PHARMACEUTICAL FORMULATIONS OF 4 , 880 , 830 A 11/ 1989 Rhodes POTASSIUM ATP CHANNEL OPENERS AND 4 ,894 , 448 A 1 / 1990 Pelzer 5 ,063 , 208 A 11/ 1991 Rosenberg et al . USES THEREOF 5 , 284 ,845 A 2 / 1994 Paulsen 5 ,356 ,775 A 10 / 1994 Hebert et al . (71 ) Applicant: Essentialis , Inc. , Carlsbad , CA (US ) 5 ,376 , 384 A 12 / 1994 Eichel et al. 5 , 378 , 704 A 1 / 1995 Weller, III (72 ) Inventor: Neil Madison Cowen , Carlsbad , CA 5 , 399 , 359 A 3 / 1995 Baichwal 5 ,415 , 871 A 5 / 1995 Pankhania et al . (US ) 5 ,629 , 045 A 5 / 1997 Veech 5 , 733 , 563 A 3 / 1998 Fortier (73 ) Assignee : Essentialis , Inc. , Redwood City , CA 5 , 744 , 594 A 4 / 1998 Adelman et al . (US ) 5 , 965 ,620 A 10 / 1999 Sorgente et al . 6 ,022 , 562 A 2 /2000 Autant et al . 6 , 140 , 343 A 10 / 2000 Deninno et al . ( * ) Notice : Subject to any disclaimer , the term of this 6 , 146 ,662 A 11/ 2000 Jao et al . patent is extended or adjusted under 35 6 , 197, 765 B1 3 / 2001 Vardi et al. U . S . C . 154 (b ) by 0 days . 6, 197, 976 B1 3 / 2001 Harrington et al . 6 , 225, 310 B1 5 / 2001 Nielsen et al. (21 ) Appl. No. : 14 / 458 ,032 6 , 255 , 459 B1 7 / 2001 Lester et al . 6 , 277, 366 B1 8 / 2001 Goto et al . -

Niflumic Acid Hyperpolarizes Smooth Muscle Cells Via Calcium-Activated Potassium Channel in Spiral Modiolar Artery of Guinea Pigs1
Acta Pharmacol Sin 2008 Jul; 29 (7): 789–799 Full-length article Niflumic acid hyperpolarizes smooth muscle cells via calcium-activated potassium channel in spiral modiolar artery of guinea pigs1 Li LI2,3, Ke-tao MA3,4, Lei ZHAO3, Jun-qiang SI3,4,5, Zhong-shuang ZHANG3, He ZHU3, Jing LI3 2Departmeng of Pharmacology, Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430030, China; 3Labo- ratory of Xinjiang Endemic and Ethnic Diseases, Shihezi University Medical College, Xinjiang 832002, China; 4Fundamental Medical School of Wuhan University, Wuhan 430071, China Key words Abstract spiral modiolar artery; smooth muscle cells; Aim: The influence of niflumic acid (NFA), a Cl– channel antagonist, on the mem- 2+ niflumic acid; hyperpolarization; Ca - brane potentials in smooth muscle cells (SMC) of the cochlear spiral modiolar activated potassium channels; cochlea artery (SMA) in guinea pigs was examined. Methods: The intracellular recording 1This work was supported by the National and whole-cell recording technique were used to record the NFA-induced re- Natural Science Foundation of China (No sponse on the acutely-isolated SMA preparation. Results: The SMC had 2 stable 30460043); the Scientific and Technological Program for Overseas Personnel, the but mutually convertible levels of resting potentials (RP), that is, one was near –45 Ministry of Personnel, China (2006); and the mV and the other was approximately –75 mV, termed as low and high RP, Key Program of Scientific and Technological respectively. The bath application of NFA could cause a hyperpolarization in all Research, the Ministry of Education, China (Local Universities; No 207134). the low RP cells, but had little effect on high RP cells. -

WO 2011/089216 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date t 28 July 2011 (28.07.2011) WO 2011/089216 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 47/48 (2006.01) C07K 1/13 (2006.01) kind of national protection available): AE, AG, AL, AM, C07K 1/1 07 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) Number: International Application DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/050821 HN, HR, HU, ID, J , IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 2 1 January 201 1 (21 .01 .201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 1015 1465. 1 22 January 2010 (22.01 .2010) EP kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (71) Applicant (for all designated States except US): AS- ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, CENDIS PHARMA AS [DK/DK]; Tuborg Boulevard TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 12, DK-2900 Hellerup (DK). -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -

KATP Channel Openers Facilitate Glutamate Uptake by Gluts in Rat Primary Cultured Astrocytes
Neuropsychopharmacology (2008) 33, 1336–1342 & 2008 Nature Publishing Group All rights reserved 0893-133X/08 $30.00 www.neuropsychopharmacology.org KATP Channel Openers Facilitate Glutamate Uptake by GluTs in Rat Primary Cultured Astrocytes 1,2 1,2 1 1 1 ,1 Xiu-Lan Sun , Xiao-Ning Zeng , Fang Zhou , Cui-Ping Dai , Jian-Hua Ding and Gang Hu* 1 Laboratory of Neuropharmacology, Department of Anatomy, Histology & Pharmacology, Nanjing Medical University, Nanjing, Jiangsu, P.R. China Increasing evidence, including from our laboratory, has revealed that opening of ATP sensitive potassium channels (KATP channels) plays the neuronal protective roles both in vivo and in vitro. Thus K channel openers (KCOs) have been proposed as potential ATP neuroprotectants. Our previous studies demonstrated that K channels could regulate glutamate uptake activity in PC12 cells as well as ATP in synaptosomes of rats. Since glutamate transporters (GluTs) of astrocytes play crucial roles in glutamate uptake and KATP channels are also expressed in astrocytes, the present study showed whether and how KATP channels regulated the function of GluTs in primary cultured astrocytes. The results showed that nonselective KCO pinacidil, selective mitochondrial KCO diazoxide, novel, and blood–brain barrier permeable KCO iptakalim could enhance glutamate uptake, except for the sarcolemmal KCO P1075. Moreover pinacidil, + diazoxide, and iptakalim reversed the inhibition of glutamate uptake induced by 1-methyl-4-phenylpyridinium (MPP ). These potentiated effects were completely abolished by mitochondrial K blocker 5-hydroxydecanoate. Furthermore, either diazoxide or iptakalim could ATP + inhibit MPP -induced elevation of reactive oxygen species (ROS) and phosphorylation of protein kinases C (PKC). These findings are the first to demonstrate that activation of KATP channel, especially mitochondrial KATP channel, improves the function of GluTs in astrocytes due to reducing ROS production and downregulating PKC phosphorylation. -

Altered ATP-Sensitive Potassium Channels May Underscore Obesity-Trig- Gered Increase in Blood Pressure1
Acta Pharmacol Sin 2008 Oct; 29 (10): 1167–1174 Full-length article Altered ATP-sensitive potassium channels may underscore obesity-trig- gered increase in blood pressure1 Li-hong FAN2,4, Hong-yan TIAN2,4, Ai-qun MA2,5, Zhi HU2, Jian-hua HUO2, Yong-xiao CAO3 2Department of Cardiology, The First Affiliated Hospital of Xi-an Jiaotong University School of Medicine, Xi-an 710061, China; 3Department of Phar- macology, Xi’an Jiaotong University School of Medicine, Xi-an 710061, China Key words Abstract ATP-sensitive potassium channel; obesity- Aim: To determine whether ATP-sensitive potassium channels are altered in hypertension; electrophysiology; aorta; VSMC from arotas and mesenteric arteries of obese rat, and their association with mesenteric artery obesity-triggered increase in blood pressure. Methods: Obesity was induced 1 Project supported by the National Natural by 24 weeks of high-fat diet feeding in male Sprague–Dawley rats. Control rats Science Foundation of China (No 30370677). were fed with standard laboratory rat chow. Blood pressure and body weight of 4 These two authors contributed equally to this work. these rats were measured every 4 weeks. At the end of 24 weeks, KATP channel- 5 Correspondence to Prof Ai-qun MA. mediated relaxation responses in the aortas and mesenteric arteries, KATP channel Phn/Fax 86-29-8526-1809. current, and gene expression were examined, respectively. Results: Blood pres- E-mail [email protected] sure and body weight were increased in rats fed with high-fat diet. KATP channel- Received 2007-12-26 mediated relaxation responses, currents, and KATP expression in VSMC of both Accepted 2008-04-22 aortas and mesenteric arteries were inhibited in these rats. -

Synthesis and Vasorelaxant Effect of 9-Aryl-1, 8-Acridinediones As
Iranian Journal of Pharmaceutical Research (2012), 11 (1): 229-233 Copyright © 2012 by School of Pharmacy Received: January 2010 Shaheed Beheshti University of Medical Sciences and Health Services Accepted: July 2010 Original Article Synthesis and Vasorelaxant Effect of 9-aryl-1,8-acridinediones as Potassium Channel Openers in Isolated Rat Aorta Mohsen Imenshahidia, Farzin Hadizadeha,b*, Asieh Firoozeh-Moghadama, Mahmoud Seifia, Atefeh Shirinbaka and Mohammad Bagher Gharedaghia aPharmacy Faculty, Mashhad University of Medical Sciences, Mashhad, Iran. bBiotechnology Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. Abstract ATP-sensitive potassium (KATP) channel openers have a relaxation effect due to the lower cellular membrane potential and inhibit calcium influx. There has been considerable interest in exploring KATP channel openers in the treatment of various diseases such as cardiovascular, cerebrovascular, and urinary system disease and premature labor. The purpose of this study was to synthesize 3,3,6,6-tetramethy l-9-aryl-octahydro-1,8-acridindiones and investigate their effects on vascular potassium channels and mechanism of induced relaxations on phenylephrine- induced contractile responses in isolated rings of rat aortic smooth muscle. In this study, four new derivatives of 3,3,6,6-tetramethy l-9-aryl-octahydro-1,8-acridindione [2a-d] were synthesized by the reaction of 5, 5-dimethyl-1,3-cyclohexanedione with an aromatic aldehyde, 2-alkylthio-1-(4-fluorobenzyl)-5-formylimidazole or 3-substituted benzaldehyde, in the presence of ammonia in methanol. Their effects on vascular potassium channels and mechanism of induced relaxations on phenylephrine-induced contractile responses in isolated rat aorta were investigated. Minoxidil was used as a standard potassium channel opener and Glibenclamide was used as a standard potassium channel blocker. -

Anti Anginal Drugs
ANTIANGINAL DRUGS Dr. BINOY VARGHESE CHERIYAN Professor Department of Pharmaceutical Chemistry Angina pectoris • Disease affecting the coronary arteries which supply oxygenated blood from left ventricle to heart tissues • The lumen of artery become restricted and it becomes less efficient in supplying the blood and oxygen to heart called as ischemia Definition of Angina GOALS OF TREATMENT • Therapy of angina is mainly directed to minimize the Anginal attacks • By restoring the balance between oxygen supply/oxygen demand to cardiac muscles or dilating coronary vessels • Reversing and preventing myocardial ischemia SUPPLY AND DEMAND. • Improve the quality of life. TYPES OF ANGINA • Stable or classical • Unstable angina or acute coronary syndrome • Prinzmetal or variant angina • What antianginal drugs do ? • Decrease the demand of oxygen or increase the supply of oxygen • Dilates coronary arteries • Decrease the after load CLASSIFICATION 1) NITRATES: a) Short acting: Glyceryl trinitrate (GTN) b) Long acting: Isosorbide dinitrate, Isosorbide mononitrate, Erythrityl tetranitrate, Pentaerythrityl tetranitrate _______________________________________________ 2) Β blockers: Metoprolol, Atenolol, Bisoprolol, Celiprolol ______________________________________________ 3) Calcium Channel Blockers: a) Verapamil, Diltiazem b) Dihydropyridine ---Nifedipine, Amlodipine, Nitrendipine, Nimodipine ________________________________________________ 4) Potassium Channel opener: Nicorandil, Pinacidil, Cromakalim, Minoxidil, Diazoxide ________________________________________________