Molecular Mechanisms Underlying Ketamine-Mediated Inhibition Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
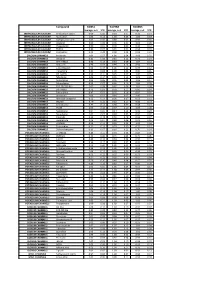
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Iatrogenic Misadventure
BRITISH MEDICAL JOURNAL 18 MARcH 1972 733 Scientific Basis of Clinical Practice Br Med J: first published as 10.1136/bmj.1.5802.733 on 18 March 1972. Downloaded from Iatrogenic Misadventure A. G. W. WHITFIELD British Medical journal, 1972, 1, 733-734 effects. No one would pretend that our knowledge of drugs interacting with oral anticoagulants is complete. Even so, A striking feature of the history of therapeutics is the extent to sufficient is already known to indicate that anticoagulant drugs which fashion, rather than clinical science, governs the treat- have potent dangers and should be used only within the limited ment we mete out to our patients. Some ten years ago I attended sphere in which they are of proven worth. Moreover, when they a symposium on anticoagulant therapy and returned home are given, if possible, no other drug should be given at the firmly convinced that in all but a very few conditions such same time as many are known to inhibit or enhance their effect treatment was absolutely essential and indeed it was little short and many others may have similar interactions, even though of criminal negligence to withhold it. At that time the patient these are as yet unrecognized and unreported. queue for "prothrombin times" was usually the longest in our hospitals and phenindione was among the most frequently prescribed drugs, but our constant use of it stemmed from enthusiasm and ignorance of its dangers and not from scientific Hypotensive Drugs proof of its therapeutic advantages. Indeed it is only as know- Hypotensive drugs are being used increasingly and undoubtedly ledge has accumulated that we have learned of the toxic effects, their development has provided an enormous advance both in of the drug we gave so freely, on the kidney, the liver, the bone the treatment and in the prognosis of the severe forms of marrow, and the skin and that except in thromboembolic disease and in patients with hypertension-particularly in the younger age groups. -

Label-Free Cell Phenotypic Profiling Decodes the Composition And
OPEN Label-free cell phenotypic profiling SUBJECT AREAS: decodes the composition and signaling POTASSIUM CHANNELS SENSORS AND PROBES of an endogenous ATP-sensitive Received potassium channel 28 January 2014 Haiyan Sun1*, Ying Wei1, Huayun Deng1, Qiaojie Xiong2{, Min Li2, Joydeep Lahiri1 & Ye Fang1 Accepted 24 April 2014 1Biochemical Technologies, Science and Technology Division, Corning Incorporated, Corning, NY 14831, United States of Published America, 2The Solomon H. Snyder Department of Neuroscience and High Throughput Biology Center, Johns Hopkins University 12 May 2014 School of Medicine, Baltimore, Maryland 21205, United States of America. Current technologies for studying ion channels are fundamentally limited because of their inability to Correspondence and functionally link ion channel activity to cellular pathways. Herein, we report the use of label-free cell requests for materials phenotypic profiling to decode the composition and signaling of an endogenous ATP-sensitive potassium should be addressed to ion channel (KATP) in HepG2C3A, a hepatocellular carcinoma cell line. Label-free cell phenotypic agonist Y.F. (fangy2@corning. profiling showed that pinacidil triggered characteristically similar dynamic mass redistribution (DMR) com) signals in A431, A549, HT29 and HepG2C3A, but not in HepG2 cells. Reverse transcriptase PCR, RNAi knockdown, and KATP blocker profiling showed that the pinacidil DMR is due to the activation of SUR2/ Kir6.2 KATP channels in HepG2C3A cells. Kinase inhibition and RNAi knockdown showed that the pinacidil * Current address: activated KATP channels trigger signaling through Rho kinase and Janus kinase-3, and cause actin remodeling. The results are the first demonstration of a label-free methodology to characterize the Biodesign Institute, composition and signaling of an endogenous ATP-sensitive potassium ion channel. -

Thiruttu Tuomittur
THIRUTTUUS009782416B2 TUOMITTUR (12 ) United States Patent ( 10 ) Patent No. : US 9 ,782 ,416 B2 Cowen (45 ) Date of Patent: Oct. 10 , 2017 ( 54 ) PHARMACEUTICAL FORMULATIONS OF 4 , 880 , 830 A 11/ 1989 Rhodes POTASSIUM ATP CHANNEL OPENERS AND 4 ,894 , 448 A 1 / 1990 Pelzer 5 ,063 , 208 A 11/ 1991 Rosenberg et al . USES THEREOF 5 , 284 ,845 A 2 / 1994 Paulsen 5 ,356 ,775 A 10 / 1994 Hebert et al . (71 ) Applicant: Essentialis , Inc. , Carlsbad , CA (US ) 5 ,376 , 384 A 12 / 1994 Eichel et al. 5 , 378 , 704 A 1 / 1995 Weller, III (72 ) Inventor: Neil Madison Cowen , Carlsbad , CA 5 , 399 , 359 A 3 / 1995 Baichwal 5 ,415 , 871 A 5 / 1995 Pankhania et al . (US ) 5 ,629 , 045 A 5 / 1997 Veech 5 , 733 , 563 A 3 / 1998 Fortier (73 ) Assignee : Essentialis , Inc. , Redwood City , CA 5 , 744 , 594 A 4 / 1998 Adelman et al . (US ) 5 , 965 ,620 A 10 / 1999 Sorgente et al . 6 ,022 , 562 A 2 /2000 Autant et al . 6 , 140 , 343 A 10 / 2000 Deninno et al . ( * ) Notice : Subject to any disclaimer , the term of this 6 , 146 ,662 A 11/ 2000 Jao et al . patent is extended or adjusted under 35 6 , 197, 765 B1 3 / 2001 Vardi et al. U . S . C . 154 (b ) by 0 days . 6, 197, 976 B1 3 / 2001 Harrington et al . 6 , 225, 310 B1 5 / 2001 Nielsen et al. (21 ) Appl. No. : 14 / 458 ,032 6 , 255 , 459 B1 7 / 2001 Lester et al . 6 , 277, 366 B1 8 / 2001 Goto et al . -

(12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau Et Al
US 20150202317A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau et al. (43) Pub. Date: Jul. 23, 2015 (54) DIPEPTDE-BASED PRODRUG LINKERS Publication Classification FOR ALPHATIC AMNE-CONTAINING DRUGS (51) Int. Cl. A647/48 (2006.01) (71) Applicant: Ascendis Pharma A/S, Hellerup (DK) A638/26 (2006.01) A6M5/9 (2006.01) (72) Inventors: Harald Rau, Heidelberg (DE); Torben A 6LX3/553 (2006.01) Le?mann, Neustadt an der Weinstrasse (52) U.S. Cl. (DE) CPC ......... A61K 47/48338 (2013.01); A61 K3I/553 (2013.01); A61 K38/26 (2013.01); A61 K (21) Appl. No.: 14/674,928 47/48215 (2013.01); A61M 5/19 (2013.01) (22) Filed: Mar. 31, 2015 (57) ABSTRACT The present invention relates to a prodrug or a pharmaceuti Related U.S. Application Data cally acceptable salt thereof, comprising a drug linker conju (63) Continuation of application No. 13/574,092, filed on gate D-L, wherein D being a biologically active moiety con Oct. 15, 2012, filed as application No. PCT/EP2011/ taining an aliphatic amine group is conjugated to one or more 050821 on Jan. 21, 2011. polymeric carriers via dipeptide-containing linkers L. Such carrier-linked prodrugs achieve drug releases with therapeu (30) Foreign Application Priority Data tically useful half-lives. The invention also relates to pharma ceutical compositions comprising said prodrugs and their use Jan. 22, 2010 (EP) ................................ 10 151564.1 as medicaments. US 2015/0202317 A1 Jul. 23, 2015 DIPEPTDE-BASED PRODRUG LINKERS 0007 Alternatively, the drugs may be conjugated to a car FOR ALPHATIC AMNE-CONTAINING rier through permanent covalent bonds. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

Niflumic Acid Hyperpolarizes Smooth Muscle Cells Via Calcium-Activated Potassium Channel in Spiral Modiolar Artery of Guinea Pigs1
Acta Pharmacol Sin 2008 Jul; 29 (7): 789–799 Full-length article Niflumic acid hyperpolarizes smooth muscle cells via calcium-activated potassium channel in spiral modiolar artery of guinea pigs1 Li LI2,3, Ke-tao MA3,4, Lei ZHAO3, Jun-qiang SI3,4,5, Zhong-shuang ZHANG3, He ZHU3, Jing LI3 2Departmeng of Pharmacology, Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430030, China; 3Labo- ratory of Xinjiang Endemic and Ethnic Diseases, Shihezi University Medical College, Xinjiang 832002, China; 4Fundamental Medical School of Wuhan University, Wuhan 430071, China Key words Abstract spiral modiolar artery; smooth muscle cells; Aim: The influence of niflumic acid (NFA), a Cl– channel antagonist, on the mem- 2+ niflumic acid; hyperpolarization; Ca - brane potentials in smooth muscle cells (SMC) of the cochlear spiral modiolar activated potassium channels; cochlea artery (SMA) in guinea pigs was examined. Methods: The intracellular recording 1This work was supported by the National and whole-cell recording technique were used to record the NFA-induced re- Natural Science Foundation of China (No sponse on the acutely-isolated SMA preparation. Results: The SMC had 2 stable 30460043); the Scientific and Technological Program for Overseas Personnel, the but mutually convertible levels of resting potentials (RP), that is, one was near –45 Ministry of Personnel, China (2006); and the mV and the other was approximately –75 mV, termed as low and high RP, Key Program of Scientific and Technological respectively. The bath application of NFA could cause a hyperpolarization in all Research, the Ministry of Education, China (Local Universities; No 207134). the low RP cells, but had little effect on high RP cells. -

USP–NF 2021, Issue 1 Commentary
Commentary USP–NF 2021 Issue 1 November 2, 2020 In accordance with USP’s Rules and Procedures of the Council of Experts (“Rules”), and except as provided in Section 9.02 Accelerated Revision Processes, USP publishes proposed revisions to the United States Pharmacopeia and the National Formulary (USP–NF) for public review and comment in the Pharmacopeial Forum (PF), USP’s free bimonthly journal for public notice and comment. After comments are considered and incorporated as the Expert Committee deems appropriate, the proposal may advance to official status or be re-published in PF for further notice and comment, in accordance with the Rules. In cases when proposals advance to official status, a summary of comments received and the appropriate Expert Committee's responses, as well as Expert Committee-initiated changes, are published in the Proposal Status/Commentary section of USPNF.com at the time the official revision is published. The Commentary is not part of the official text and is not intended to be enforceable by regulatory authorities. Rather, it explains the basis of Expert Committees’ responses to public comments on proposed revisions. If there is a difference or conflict between the contents of the Commentary and the official text, the official text prevails. For further information, contact: USP Executive Secretariat United States Pharmacopeia 12601 Twinbrook Parkway Rockville, MD 20852-1790 USA Commentary for USP–NF 2021, Issue 1 Comments were received for the following when they were proposed in Pharmacopeial Forum: General -

Chapter 260-70 WAC EQUINE MEDICATION PROGRAM
Chapter 260-70 Chapter 260-70 WAC EQUINE MEDICATION PROGRAM WAC 12/6/93. Statutory Authority: RCW 67.16.020 and 260-70-500 Definitions applicable to chapter 260-70 WAC. 67.16.040. 84-06-061 (Order 84-01), § 260-70-028, 260-70-510 Equine health and safety. filed 3/7/84.] Repealed by 96-10-001, filed 4/17/96, 260-70-540 Veterinarians' reports. effective 5/18/96. Statutory Authority: RCW 260-70-545 Prohibited practices. 67.16.040. 260-70-550 Medication labeling. 260-70-029 Receiving barn. [Statutory Authority: RCW 67.16.020 260-70-560 Treatment restrictions. and 67.16.040. 84-06-061 (Order 84-01), § 260-70-029, 260-70-570 All horses are subject to inspection. filed 3/7/84.] Repealed by 96-10-001, filed 4/17/96, 260-70-580 Official veterinarian's list. effective 5/18/96. Statutory Authority: RCW 67.16.- 260-70-590 Reporting to the test barn. 040. 260-70-600 Sample collection. 260-70-030 When administration prohibited. [Order 74.1, § 260-70- 260-70-610 Storage and shipment of split samples. 030, filed 5/22/74, effective 7/1/74.] Repealed by 80-01- 260-70-620 Medication restrictions. 072 (Order 79-02), filed 12/24/79. Statutory Authority: 260-70-630 Threshold levels. RCW 67.16.020 and 67.16.040. 260-70-640 Permitted medication. 260-70-031 Reporting to receiving barn. [Statutory Authority: 260-70-645 Anti-ulcer medications. RCW 67.16.020 and 67.16.040. 84-06-061 (Order 84- 260-70-650 Furosemide. -

WO 2011/089216 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date t 28 July 2011 (28.07.2011) WO 2011/089216 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 47/48 (2006.01) C07K 1/13 (2006.01) kind of national protection available): AE, AG, AL, AM, C07K 1/1 07 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) Number: International Application DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/050821 HN, HR, HU, ID, J , IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 2 1 January 201 1 (21 .01 .201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 1015 1465. 1 22 January 2010 (22.01 .2010) EP kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (71) Applicant (for all designated States except US): AS- ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, CENDIS PHARMA AS [DK/DK]; Tuborg Boulevard TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 12, DK-2900 Hellerup (DK). -

Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla
2006년 2월 박사학위논문 Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla 조선대학교 대학원 의학과 이은숙 Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla 흰쥐 부신수질에서 니코란딜의 카테콜아민 분비작용에 대한 억제기전 2006년 2월 일 조선대학교 대학원 의학과 이은숙 Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla 지도교수 임임임 동동동 윤윤윤 이이이 논문을 의학박사 학위 신청논문으로 제출함. 2005 년 10 월 일 조조조선대학교조선대학교 대학원 의학과 이은숙 이 은 숙의 박사학위논문을 인준함 위원장 서울 대학교 교수 인인인 위위위 원원원 전남 대학교 교수 인인인 위위위 원원원 조선 대학교 교수 인인인 위위위 원원원 조선 대학교 교수 인인인 위위위 원원원 조선 대학교 교수 인인인 2005 년 12 월 29 일 조선대학교 대학원 CONTENTS KOREAN ABSTRACT ---------------------------------------------------------------------- I. INTRODUCTION ---------------------------------------------------------------------------- II. MATERIALS AND METHODS ----------------------------------------------------------- Experimental Procedure ------------------------------------------------------------------------------ Perfusion of Adrenal Gland -------------------------------------------------------------------------- Drug Administration --------------------------------- -------------------------------------------------- Collection of Perfusate -------------------------------------------------------------------------------- Measurement of Catecholamines ------------------------------------------------------------------ Statistical Analysis ------------------------------------------------------------------------------------- Drugs and Their Sources -----------------------------------------------------------------------------