Niflumic Acid Hyperpolarizes Smooth Muscle Cells Via Calcium-Activated Potassium Channel in Spiral Modiolar Artery of Guinea Pigs1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Hypercholesterolaemia on Voltage-Operated Calcium Channel Currents in Rabbit Arterial Smooth Muscle Cells
Journal of Human Hypertension (1999) 13, 849–853 1999 Stockton Press. All rights reserved 0950-9240/99 $15.00 http://www.stockton-press.co.uk/jhh Effect of hypercholesterolaemia on voltage-operated calcium channel currents in rabbit arterial smooth muscle cells GF Clunn, S Wijetunge and AD Hughes Clinical Pharmacology, NHLI, St. Mary’s Hospital, Imperial College of Science, Technology and Medicine, South Wharf Road, London, W2 1NY, UK Cholesterol is a major component of cell membranes capacitance was also greater in NZ cells. Consequently, and influences membrane fluidity. Watanabe heritable there was no significant difference in current density hyperpercholesterolaemic rabbits (WHHL) possess between NZ and WHHL cells either in the absence of defective receptors for low density lipoprotein leading drug or in the presence of the calcium channel agonist to increased plasma cholesterol, accumulation of chol- (+)202 791. Current voltage-relationships, kinetics of esterol in the arterial wall and atherosclerosis. In this fast inactivation and steady-state inactivation of IBa also study calcium channel currents (IBa) were compared did not differ significantly between WHHL and NZ. These using conventional whole cell voltage clamp techniques findings suggest that hypercholesterolaemia in WHHL in ear artery cells isolated from control New Zealand has no direct effect on calcium channel current density White rabbits (NZ) with those from WHHL. IBa were larger or voltage-modulation in arterial smooth muscle cells. in cells isolated from NZ than from WHHL, however cell Keywords: calcium channel; cholesterol; vascular smooth muscle; Watanabe hypercholesterolaemic rabbit Introduction atic cholesterol toxicity or other organ damage which develops in cholesterol-fed rabbits.15 WHHL Cholesterol is a major component of cell membranes 1,2 has therefore been proposed to be a model of human and influences membrane structure and fluidity. -

Molecular Mechanisms Underlying Ketamine-Mediated Inhibition Of
Anesthesiology 2005; 102:93–101 © 2004 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Molecular Mechanisms Underlying Ketamine-mediated Inhibition of Sarcolemmal Adenosine Triphosphate- sensitive Potassium Channels Takashi Kawano, M.D.,* Shuzo Oshita, M.D.,† Akira Takahashi, M.D.,‡ Yasuo Tsutsumi, M.D.,* Katsuya Tanaka, M.D.,§ Yoshinobu Tomiyama, M.D., Hiroshi Kitahata, M.D.,# Yutaka Nakaya, M.D.** Background: Ketamine inhibits adenosine triphosphate-sen- sensitive channel pore.3 These channels, as metabolic sitive potassium (KATP) channels, which results in the blocking sensors, are associated with such cellular functions as of ischemic preconditioning in the heart and inhibition of va- insulin secretion, cardiac preconditioning, vasodilata- sorelaxation induced by KATP channel openers. In the current 4–7 Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/102/1/93/357819/0000542-200501000-00017.pdf by guest on 27 September 2021 study, the authors investigated the molecular mechanisms of tion, and neuroprotection. ketamine’s actions on sarcolemmal KATP channels that are re- In cardiac myocytes, intravenous general anesthetics, associated by expressed subunits, inwardly rectifying potas- such as ketamine racemate, propofol, and thiamylal, di- sium channels (Kir6.1 or Kir6.2) and sulfonylurea receptors 8–10 rectly inhibit native sarcolemmal KATP channels. Al- (SUR1, SUR2A, or SUR2B). though these observations suggest that intravenous an- Methods: The authors used inside-out patch clamp configura- tions to investigate the effects of ketamine on the activities of esthetics may impair the endogenous organ protective reassociated Kir6.0/SUR channels containing wild-type, mutant, mechanisms mediated by KATP channels, the possibility or chimeric SURs expressed in COS-7 cells. -
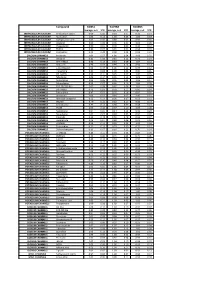
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Label-Free Cell Phenotypic Profiling Decodes the Composition And
OPEN Label-free cell phenotypic profiling SUBJECT AREAS: decodes the composition and signaling POTASSIUM CHANNELS SENSORS AND PROBES of an endogenous ATP-sensitive Received potassium channel 28 January 2014 Haiyan Sun1*, Ying Wei1, Huayun Deng1, Qiaojie Xiong2{, Min Li2, Joydeep Lahiri1 & Ye Fang1 Accepted 24 April 2014 1Biochemical Technologies, Science and Technology Division, Corning Incorporated, Corning, NY 14831, United States of Published America, 2The Solomon H. Snyder Department of Neuroscience and High Throughput Biology Center, Johns Hopkins University 12 May 2014 School of Medicine, Baltimore, Maryland 21205, United States of America. Current technologies for studying ion channels are fundamentally limited because of their inability to Correspondence and functionally link ion channel activity to cellular pathways. Herein, we report the use of label-free cell requests for materials phenotypic profiling to decode the composition and signaling of an endogenous ATP-sensitive potassium should be addressed to ion channel (KATP) in HepG2C3A, a hepatocellular carcinoma cell line. Label-free cell phenotypic agonist Y.F. (fangy2@corning. profiling showed that pinacidil triggered characteristically similar dynamic mass redistribution (DMR) com) signals in A431, A549, HT29 and HepG2C3A, but not in HepG2 cells. Reverse transcriptase PCR, RNAi knockdown, and KATP blocker profiling showed that the pinacidil DMR is due to the activation of SUR2/ Kir6.2 KATP channels in HepG2C3A cells. Kinase inhibition and RNAi knockdown showed that the pinacidil * Current address: activated KATP channels trigger signaling through Rho kinase and Janus kinase-3, and cause actin remodeling. The results are the first demonstration of a label-free methodology to characterize the Biodesign Institute, composition and signaling of an endogenous ATP-sensitive potassium ion channel. -

Thiruttu Tuomittur
THIRUTTUUS009782416B2 TUOMITTUR (12 ) United States Patent ( 10 ) Patent No. : US 9 ,782 ,416 B2 Cowen (45 ) Date of Patent: Oct. 10 , 2017 ( 54 ) PHARMACEUTICAL FORMULATIONS OF 4 , 880 , 830 A 11/ 1989 Rhodes POTASSIUM ATP CHANNEL OPENERS AND 4 ,894 , 448 A 1 / 1990 Pelzer 5 ,063 , 208 A 11/ 1991 Rosenberg et al . USES THEREOF 5 , 284 ,845 A 2 / 1994 Paulsen 5 ,356 ,775 A 10 / 1994 Hebert et al . (71 ) Applicant: Essentialis , Inc. , Carlsbad , CA (US ) 5 ,376 , 384 A 12 / 1994 Eichel et al. 5 , 378 , 704 A 1 / 1995 Weller, III (72 ) Inventor: Neil Madison Cowen , Carlsbad , CA 5 , 399 , 359 A 3 / 1995 Baichwal 5 ,415 , 871 A 5 / 1995 Pankhania et al . (US ) 5 ,629 , 045 A 5 / 1997 Veech 5 , 733 , 563 A 3 / 1998 Fortier (73 ) Assignee : Essentialis , Inc. , Redwood City , CA 5 , 744 , 594 A 4 / 1998 Adelman et al . (US ) 5 , 965 ,620 A 10 / 1999 Sorgente et al . 6 ,022 , 562 A 2 /2000 Autant et al . 6 , 140 , 343 A 10 / 2000 Deninno et al . ( * ) Notice : Subject to any disclaimer , the term of this 6 , 146 ,662 A 11/ 2000 Jao et al . patent is extended or adjusted under 35 6 , 197, 765 B1 3 / 2001 Vardi et al. U . S . C . 154 (b ) by 0 days . 6, 197, 976 B1 3 / 2001 Harrington et al . 6 , 225, 310 B1 5 / 2001 Nielsen et al. (21 ) Appl. No. : 14 / 458 ,032 6 , 255 , 459 B1 7 / 2001 Lester et al . 6 , 277, 366 B1 8 / 2001 Goto et al . -

Siteand Mechanism of Action of Resin Acids on Voltage-Gated Ion Channels
Linköping University Medical Dissertation No. 1620 Site and Mechanism of Action of Resin Acids on Voltage-Gated Ion Channels Malin Silverå Ejneby Department of Clinical and Experimental Medicine Linköping University, Sweden Linköping 2018 © Malin Silverå Ejneby, 2018 Cover illustration: “The Charged Pine Tree Anchored to the Ground” was designed and painted by Daniel Silverå Ejneby. Printed in Sweden by LiU-Tryck, Linköping, Sweden, 2018 ISSN: 0345-0082 ISBN: 978-91-7685-318-4 TABLE OF CONTENTS ABSTRACT ............................................................................................................................... 1 POPULÄRVETENSKAPLIG SAMMANFATTNING ................................................................. 3 LIST OF ARTICLES .................................................................................................................. 5 INTRODUCTION ....................................................................................................................... 7 Ions underlie the electrical activity in the heart and brain .................................................. 7 A mathematical model for the nerve impulse - Hodgkin and Huxley ............................... 7 Cardiac action potentials – a great diversity of shapes ...................................................... 9 Voltage-gated ion channels ......................................................................................................... 9 General structure .................................................................................................................... -

WO 2011/089216 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date t 28 July 2011 (28.07.2011) WO 2011/089216 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 47/48 (2006.01) C07K 1/13 (2006.01) kind of national protection available): AE, AG, AL, AM, C07K 1/1 07 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) Number: International Application DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/050821 HN, HR, HU, ID, J , IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 2 1 January 201 1 (21 .01 .201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 1015 1465. 1 22 January 2010 (22.01 .2010) EP kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (71) Applicant (for all designated States except US): AS- ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, CENDIS PHARMA AS [DK/DK]; Tuborg Boulevard TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 12, DK-2900 Hellerup (DK). -

Potentiation of Large Conductance Kca Channels by Niflumic, Flufenamic, and Mefenamic Acids
2272 Biophysical Journal Volume 67 December 1994 2272-2279 Potentiation of Large Conductance KCa Channels by Niflumic, Flufenamic, and Mefenamic Acids M. Ottolia and L. Toro Department of Molecular Physiology and Biophysics, Baylor College of Medicine, Houston, Texas 77030 USA ABSTRACT Large conductance calcium-activated K+ (Kca) channels are rapidly activated by niflumic acid dose- dependently and reversibly. External niflumic acid was about 5 times more potent than internal niflumic acid, and its action was characterized by an increase in the channel affinity for [Ca2l], a parallel left shift of the voltage-activation curve, and a decrease of the channel long-closed states. Niflumic acid applied from the external side did not interfere with channel block by charybdotoxin, suggesting that its site of action is not at or near the charybdotoxin receptor. Accordingly, partial tetraethylammonium blockade did not interfere with channel activation by niflumic acid. Flufenamic acid and mefenamic acid also stimulated KCa channel activity and, as niflumic acid, they were more potent from the external than from the internal side. Fenamates applied from the external side displayed the following potency sequence: flufenamic acid niflumic acid >> mefenamic acid. These results indicate that KCa channels possess at least one fenamatereceptor whose occupancy leads to channel opening. INTRODUCTION Large conductance calcium-activated K channels are present quently purified in a sucrose gradient. Membranes obtained from the of cell types (Latorre et al., 1989; McManus, 1991). 20%:25% and 25%:30% (w/w) sucrose interface were used. Lipid bi- in a variety layers were cast from a phospholipid solution in n-decane containing a In neurons they may regulate cell firing (Gola and Crest, 1993), 5:2:3 mixture of phosphatidylethanolamine/phosphatidylserine/phos- and in smooth muscle they seem to play an important role in phatidylcholine (25 mg/ml). -

Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla
2006년 2월 박사학위논문 Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla 조선대학교 대학원 의학과 이은숙 Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla 흰쥐 부신수질에서 니코란딜의 카테콜아민 분비작용에 대한 억제기전 2006년 2월 일 조선대학교 대학원 의학과 이은숙 Inhibitory Mechanism of Nicorandil on Catecholamine Secretion from the Rat Adrenal Medulla 지도교수 임임임 동동동 윤윤윤 이이이 논문을 의학박사 학위 신청논문으로 제출함. 2005 년 10 월 일 조조조선대학교조선대학교 대학원 의학과 이은숙 이 은 숙의 박사학위논문을 인준함 위원장 서울 대학교 교수 인인인 위위위 원원원 전남 대학교 교수 인인인 위위위 원원원 조선 대학교 교수 인인인 위위위 원원원 조선 대학교 교수 인인인 위위위 원원원 조선 대학교 교수 인인인 2005 년 12 월 29 일 조선대학교 대학원 CONTENTS KOREAN ABSTRACT ---------------------------------------------------------------------- I. INTRODUCTION ---------------------------------------------------------------------------- II. MATERIALS AND METHODS ----------------------------------------------------------- Experimental Procedure ------------------------------------------------------------------------------ Perfusion of Adrenal Gland -------------------------------------------------------------------------- Drug Administration --------------------------------- -------------------------------------------------- Collection of Perfusate -------------------------------------------------------------------------------- Measurement of Catecholamines ------------------------------------------------------------------ Statistical Analysis ------------------------------------------------------------------------------------- Drugs and Their Sources ----------------------------------------------------------------------------- -

Molecular Properties of Drugs Interacting with SLC22 Transporters OAT1, OAT3, OCT1, and OCT2: a Machine-Learning Approach S
Supplemental material to this article can be found at: http://jpet.aspetjournals.org/content/suppl/2016/08/03/jpet.116.232660.DC1 1521-0103/359/1/215–229$25.00 http://dx.doi.org/10.1124/jpet.116.232660 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 359:215–229, October 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics Molecular Properties of Drugs Interacting with SLC22 Transporters OAT1, OAT3, OCT1, and OCT2: A Machine-Learning Approach s Henry C. Liu, Anne Goldenberg, Yuchen Chen, Christina Lun, Wei Wu, Kevin T. Bush, Natasha Balac, Paul Rodriguez, Ruben Abagyan, and Sanjay K. Nigam Departments of Bioengineering (H.C.L.), Pediatrics (A.G., Y.C., C.L., K.T.B., S.K.N.), Medicine (W.W., S.K.N.), Cellular and Molecular Medicine (S.K.N.), and Pharmacology (R.A.), and the San Diego Supercomputer Center (N.B., P.R.), University of California San Diego, La Jolla, California Downloaded from Received February 2, 2016; accepted July 20, 2016 ABSTRACT Statistical analysis was performed on physicochemical descrip- ligands; this was confirmed by quantitative atomic property field tors of ∼250 drugs known to interact with one or more SLC22 analysis. Virtual screening with this pharmacophore, followed by “drug” transporters (i.e., SLC22A6 or OAT1, SLC22A8 or OAT3, transport assays, identified several cationic drugs that selectively jpet.aspetjournals.org SLC22A1 or OCT1, and SLC22A2 or OCT2), followed by applica- interact with OAT3 but not OAT1. Although the present analysis tion of machine-learning methods and wet laboratory testing of may be somewhat limited by the need to rely largely on inhibition novel predictions. -

Live Horses, Asses, Mules, and Hinnies: - - Horses: 0101.21.0000 - - - Purebred Breeding Animals No
Schedule B No. Second Commodity Description Unit of and Headings Quantity Quantity 01 Live Animals 0101 - Live horses, asses, mules, and hinnies: - - Horses: 0101.21.0000 - - - Purebred breeding animals No. 0101.29.0000 - - - Other No. 0101.30.0000 - - Asses No. 0101.90.0000 - - Other No. 0102 - Live bovine animals: - - Cattle: 0102.21 - - - Purebred breeding animals: - - - - Dairy: 0102.21.0010 - - - - - Male No. 0102.21.0020 - - - - - Female No. - - - - Other: 0102.21.0030 - - - - - Male No. 0102.21.0050 - - - - - Female No. 0102.29.0000 - - - Other No. - - Buffalo: 0102.31.0000 - - - Purebred breeding animals No. 0102.39.0000 - - - Other No. 0102.90.0002 - - Other No. 0103 - Live swine: 0103.10.0000 - - Purebred breeding animals No. - - Other: 0103.91.0000 - - - Weighing less than 50 kg (110.23 lb.) each No. 0103.92.0000 - - - Weighing 50 kg (110.23 lb.) or more each No. 0104 - Live sheep and goats: 0104.10.0000 - - Sheep No. 0104.20.0000 - - Goats No. 0105 - Live poultry of the following kinds: chickens, ducks, geese, turkeys and guineas: - - Weighing not more than 185 g (6.53 oz.) each: 0105.11 - - - Chickens: - - - - Breeding stock, whether or not purebred: 0105.11.0010 - - - - - Layer-type (egg-type) No. 0105.11.0020 - - - - - Broiler-type (meat-type) No. 0105.11.0040 - - - - Other No. 0105.12.0000 - - - Turkeys No. 0105.13.0000 - - - Ducks No. 0105.14.0000 - - - Geese No. 0105.15.0000 - - - Guinea fowls No. - - Other: 0105.94.0000 - - - Chickens No. Schedule B No. Second Commodity Description Unit of and Headings Quantity Quantity 0105.99.0000 - - - Other No. 0106 - Other live animals: - - Mammals: 0106.11.0000 - - - Primates No. 0106.12.0100 - - - Whales, dolphins and porpoises (mammals of the order Cetacea); manatees and dugongs (mammals of the order Sirenia); seals, sea lions and walruses (mammals of the suborder Pinnipedia) No.