Label-Free Cell Phenotypic Profiling Decodes the Composition And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Mechanisms Underlying Ketamine-Mediated Inhibition Of
Anesthesiology 2005; 102:93–101 © 2004 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Molecular Mechanisms Underlying Ketamine-mediated Inhibition of Sarcolemmal Adenosine Triphosphate- sensitive Potassium Channels Takashi Kawano, M.D.,* Shuzo Oshita, M.D.,† Akira Takahashi, M.D.,‡ Yasuo Tsutsumi, M.D.,* Katsuya Tanaka, M.D.,§ Yoshinobu Tomiyama, M.D., Hiroshi Kitahata, M.D.,# Yutaka Nakaya, M.D.** Background: Ketamine inhibits adenosine triphosphate-sen- sensitive channel pore.3 These channels, as metabolic sitive potassium (KATP) channels, which results in the blocking sensors, are associated with such cellular functions as of ischemic preconditioning in the heart and inhibition of va- insulin secretion, cardiac preconditioning, vasodilata- sorelaxation induced by KATP channel openers. In the current 4–7 Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/102/1/93/357819/0000542-200501000-00017.pdf by guest on 27 September 2021 study, the authors investigated the molecular mechanisms of tion, and neuroprotection. ketamine’s actions on sarcolemmal KATP channels that are re- In cardiac myocytes, intravenous general anesthetics, associated by expressed subunits, inwardly rectifying potas- such as ketamine racemate, propofol, and thiamylal, di- sium channels (Kir6.1 or Kir6.2) and sulfonylurea receptors 8–10 rectly inhibit native sarcolemmal KATP channels. Al- (SUR1, SUR2A, or SUR2B). though these observations suggest that intravenous an- Methods: The authors used inside-out patch clamp configura- tions to investigate the effects of ketamine on the activities of esthetics may impair the endogenous organ protective reassociated Kir6.0/SUR channels containing wild-type, mutant, mechanisms mediated by KATP channels, the possibility or chimeric SURs expressed in COS-7 cells. -
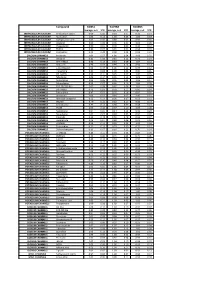
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

Topically Applied Minoxidil in Baldness
Review Topically Applied Minoxidil in Baldness ERVIN NOVAK,M.D., THOMASJ. FRANZ, M.D., JOHN T. HEADINGTON,M.D., AND RONALD C. WESTER,PH.D. From the Upjohn Company, Kalamazoo, Michigan, School idil and its metabolites can be removed by hemodi- of Medicine, University of Washington, Seattle, alysis. Washington, Medical School, University of Michigan, Ann Fluid retention and hypertrichosis are the most Arbor, Michigan, and School of Medicine and School of Pharmacy, University of California, San Francisco, California commonly occurring side effects of minoxidil. Hair regrowth in a patient with male pattern baldness was described in a case report of a hypertensive patient treated twice daily with 20 mg oral minoxidil.6 The Minoxidil, an orally administered, peripheral va- extensive hair regrowth continued after 10 months sodilator used to treat hypertension, causes hypertri- of therapy. New and increased hair growth as a side chosis in more than 80% of users. The drug reduces effect was also detected in an early clinical study elevated blood pressure by decreasing peripheral involving five of eight patients on oral minoxidil vascular resistance. Chemically, minoxidil is 2,4-di- therapy for 2 month^.^ Zappacosta' reported reversal amino-6-piperidinopyrimidine-3-oxide; it is soluble in of baldness in a patient on minoxidil for the treatment water to the extent of approximately 2 mg/ml, is of hypertension. more readily soluble in propylene glycol or ethanol, After the third week of therapy with oral minoxidil, and is nearly insoluble in acetone, chloroform, or hypertrichosis usually appears between the eyebrows ethyl acetate. Following oral administration of minox- and the hair line, in the malar and temporal areas, idil and in association with the reduction in peripheral on the backs of the arms, on the shoulders, and on vascular resistance, cardiac output is augmented, salt the legs. -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Targeting Lysophosphatidic Acid in Cancer: the Issues in Moving from Bench to Bedside
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by IUPUIScholarWorks cancers Review Targeting Lysophosphatidic Acid in Cancer: The Issues in Moving from Bench to Bedside Yan Xu Department of Obstetrics and Gynecology, Indiana University School of Medicine, 950 W. Walnut Street R2-E380, Indianapolis, IN 46202, USA; [email protected]; Tel.: +1-317-274-3972 Received: 28 August 2019; Accepted: 8 October 2019; Published: 10 October 2019 Abstract: Since the clear demonstration of lysophosphatidic acid (LPA)’s pathological roles in cancer in the mid-1990s, more than 1000 papers relating LPA to various types of cancer were published. Through these studies, LPA was established as a target for cancer. Although LPA-related inhibitors entered clinical trials for fibrosis, the concept of targeting LPA is yet to be moved to clinical cancer treatment. The major challenges that we are facing in moving LPA application from bench to bedside include the intrinsic and complicated metabolic, functional, and signaling properties of LPA, as well as technical issues, which are discussed in this review. Potential strategies and perspectives to improve the translational progress are suggested. Despite these challenges, we are optimistic that LPA blockage, particularly in combination with other agents, is on the horizon to be incorporated into clinical applications. Keywords: Autotaxin (ATX); ovarian cancer (OC); cancer stem cell (CSC); electrospray ionization tandem mass spectrometry (ESI-MS/MS); G-protein coupled receptor (GPCR); lipid phosphate phosphatase enzymes (LPPs); lysophosphatidic acid (LPA); phospholipase A2 enzymes (PLA2s); nuclear receptor peroxisome proliferator-activated receptor (PPAR); sphingosine-1 phosphate (S1P) 1. -

Iatrogenic Misadventure
BRITISH MEDICAL JOURNAL 18 MARcH 1972 733 Scientific Basis of Clinical Practice Br Med J: first published as 10.1136/bmj.1.5802.733 on 18 March 1972. Downloaded from Iatrogenic Misadventure A. G. W. WHITFIELD British Medical journal, 1972, 1, 733-734 effects. No one would pretend that our knowledge of drugs interacting with oral anticoagulants is complete. Even so, A striking feature of the history of therapeutics is the extent to sufficient is already known to indicate that anticoagulant drugs which fashion, rather than clinical science, governs the treat- have potent dangers and should be used only within the limited ment we mete out to our patients. Some ten years ago I attended sphere in which they are of proven worth. Moreover, when they a symposium on anticoagulant therapy and returned home are given, if possible, no other drug should be given at the firmly convinced that in all but a very few conditions such same time as many are known to inhibit or enhance their effect treatment was absolutely essential and indeed it was little short and many others may have similar interactions, even though of criminal negligence to withhold it. At that time the patient these are as yet unrecognized and unreported. queue for "prothrombin times" was usually the longest in our hospitals and phenindione was among the most frequently prescribed drugs, but our constant use of it stemmed from enthusiasm and ignorance of its dangers and not from scientific Hypotensive Drugs proof of its therapeutic advantages. Indeed it is only as know- Hypotensive drugs are being used increasingly and undoubtedly ledge has accumulated that we have learned of the toxic effects, their development has provided an enormous advance both in of the drug we gave so freely, on the kidney, the liver, the bone the treatment and in the prognosis of the severe forms of marrow, and the skin and that except in thromboembolic disease and in patients with hypertension-particularly in the younger age groups. -

Metabolite Sensing Gpcrs: Promising Therapeutic Targets for Cancer Treatment?
cells Review Metabolite Sensing GPCRs: Promising Therapeutic Targets for Cancer Treatment? Jesús Cosín-Roger 1,*, Dolores Ortiz-Masia 2 , Maria Dolores Barrachina 3 and Sara Calatayud 3 1 Hospital Dr. Peset, Fundación para la Investigación Sanitaria y Biomédica de la Comunitat Valenciana, FISABIO, 46017 Valencia, Spain 2 Departament of Medicine, Faculty of Medicine, University of Valencia, 46010 Valencia, Spain; [email protected] 3 Departament of Pharmacology and CIBER, Faculty of Medicine, University of Valencia, 46010 Valencia, Spain; [email protected] (M.D.B.); [email protected] (S.C.) * Correspondence: [email protected]; Tel.: +34-963851234 Received: 30 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: G-protein-coupled receptors constitute the most diverse and largest receptor family in the human genome, with approximately 800 different members identified. Given the well-known metabolic alterations in cancer development, we will focus specifically in the 19 G-protein-coupled receptors (GPCRs), which can be selectively activated by metabolites. These metabolite sensing GPCRs control crucial processes, such as cell proliferation, differentiation, migration, and survival after their activation. In the present review, we will describe the main functions of these metabolite sensing GPCRs and shed light on the benefits of their potential use as possible pharmacological targets for cancer treatment. Keywords: G-protein-coupled receptor; metabolite sensing GPCR; cancer 1. Introduction G-protein-coupled receptors (GPCRs) are characterized by a seven-transmembrane configuration, constitute the largest and most ubiquitous family of plasma membrane receptors, and regulate virtually all known physiological processes in humans [1,2]. This family includes almost one thousand genes that were initially classified on the basis of sequence homology into six classes (A–F), where classes D and E were not found in vertebrates [3]. -

G Protein-Coupled Receptors in Stem Cell Maintenance and Somatic Reprogramming to Pluripotent Or Cancer Stem Cells
BMB Reports - Manuscript Submission Manuscript Draft Manuscript Number: BMB-14-250 Title: G protein-coupled receptors in stem cell maintenance and somatic reprogramming to pluripotent or cancer stem cells Article Type: Mini Review Keywords: G protein-coupled receptors; stem cell maintenance; somatic reprogramming; cancer stem cells; pluripotent stem cell Corresponding Author: Ssang-Goo Cho Authors: Ssang-Goo Cho1,*, Hye Yeon Choi1, Subbroto Kumar Saha1, Kyeongseok Kim1, Sangsu Kim1, Gwang-Mo Yang1, BongWoo Kim1, Jin-hoi Kim1 Institution: 1Department of Animal Biotechnology, Animal Resources Research Center, and Incurable Disease Animal Model and Stem Cell Institute (IDASI), Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 143-701, Republic of Korea, UNCORRECTED PROOF 1 G protein-coupled receptors in stem cell maintenance and somatic reprogramming to 2 pluripotent or cancer stem cells 3 4 Hye Yeon Choi, Subbroto Kumar Saha, Kyeongseok Kim, Sangsu Kim, Gwang-Mo 5 Yang, BongWoo Kim, Jin-hoi Kim, and Ssang-Goo Cho 6 7 Department of Animal Biotechnology, Animal Resources Research Center, and 8 Incurable Disease Animal Model and Stem Cell Institute (IDASI), Konkuk University, 9 120 Neungdong-ro, Gwangjin-gu, Seoul 143-701, Republic of Korea 10 * 11 Address correspondence to Ssang-Goo Cho, Department of Animal Biotechnology and 12 Animal Resources Research Center. Konkuk University, 120 Neungdong-ro, Gwangjin- 13 gu, Seoul 143-701, Republic of Korea. Tel: 82-2-450-4207, Fax: 82-2-450-1044, E-mail: 14 [email protected] 15 16 17 18 19 1 UNCORRECTED PROOF 20 Abstract 21 The G protein-coupled receptors (GPCRs) compose the third largest gene family in the 22 human genome, representing more than 800 distinct genes and 3–5% of the human genome. -

Acquired Hypertrichosis of the Periorbital Area and Malar Cheek
PHOTO CHALLENGE Acquired Hypertrichosis of the Periorbital Area and Malar Cheek Caitlin G. Purvis, BS; Justin P. Bandino, MD; Dirk M. Elston, MD An otherwise healthy woman in her late 50s with Fitzpatrick skin type II presented to the derma- tology department for a scheduled cosmetic botulinum toxin injection. Her medical history was notable only for periodic nonsurgical cosmetic procedures including botulinum toxin and dermal fillers, and she was not taking any daily systemic medications. Duringcopy the preoperative assess- ment, subtle bilateral and symmetric hypertricho- sis with darker terminal hair formation was noted on the periorbital skin and zygomatic cheek. Uponnot inquiry, the patient admitted to purchas- ing a “special eye drop” from Mexico and using it regularly. After instillation of 2 to 3 drops per eye, she would laterally wipe the resulting excess Dodrops away from the eyes with her hands and then wash her hands. She denied a change in eye color from their natural brown but did report using blue color contact lenses. She denied an increase in hair growth elsewhere including the upper lip, chin, upper chest, forearms, and hands. She denied deepening of her voice, CUTIS acne, or hair thinning. WHAT’S THE DIAGNOSIS? a. acetazolamide-induced hypertrichosis b. betamethasone-induced hypertrichosis c. bimatoprost-induced hypertrichosis d. cyclosporine-induced hypertrichosis e. timolol-induced hypertrichosis PLEASE TURN TO PAGE E21 FOR THE DIAGNOSIS From the Department of Dermatology, Medical University of South Carolina, Charleston. The authors report no conflict of interest. Correspondence: Justin P. Bandino, MD, 171 Ashley Ave, MSC 908, Charleston, SC 29425 ([email protected]). -

G Protein-Coupled Receptor 35: an Emerging Target in Inflammatory and Cardiovascular Disease
Divorty, N., Mackenzie, A., Nicklin, S., and Milligan, G. (2015) G protein- coupled receptor 35: an emerging target in inflammatory and cardiovascular disease. Frontiers in Pharmacology, 6, 41. Copyright © 2015 The Authors. This work is made available under the Creative Commons Attribution 4.0 International License (CC BY 4.0) Version: Published http://eprints.gla.ac.uk/106259/ Deposited on: 19 May 2015 Enlighten – Research publications by members of the University of Glasgow http://eprints.gla.ac.uk REVIEW ARTICLE published: 10 March 2015 doi: 10.3389/fphar.2015.00041 G protein-coupled receptor 35: an emerging target in inflammatory and cardiovascular disease Nina Divorty1,2 †, Amanda E. Mackenzie1†, Stuart A. Nicklin 2 and Graeme Milligan1* 1 Molecular Pharmacology Group, Institute of Molecular, Cell, and Systems Biology, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK 2 Institute of Cardiovascular and Medical Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK Edited by: G protein-coupled receptor 35 (GPR35) is an orphan receptor, discovered in 1998, that has Terry Kenakin, University of North garnered interest as a potential therapeutic target through its association with a range of Carolina at Chapel Hill, USA diseases. However, a lack of pharmacological tools and the absence of convincingly defined Reviewed by: endogenous ligands have hampered the understanding of function necessary to exploit it Domenico Criscuolo, Genovax, Italy J. M. Ad Sitsen, ClinPharMed, therapeutically. Although several endogenous molecules can activate GPR35 none has yet Netherlands been confirmed as the key endogenous ligand due to reasons that include lack of biological *Correspondence: specificity, non-physiologically relevant potency and species ortholog selectivity. -

Integrated Approaches for Genome-Wide Interrogation of The
MINIREVIEW THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 290, NO. 32, pp. 19471–19477, August 7, 2015 © 2015 by The American Society for Biochemistry and Molecular Biology, Inc. Published in the U.S.A. 5-HT serotonin receptor agonism have long been docu- Integrated Approaches for 2B mented to induce severe, life-threatening valvular heart disease Genome-wide Interrogation of (6–8). Indeed, based on the potent 5-HT2B agonist activity of the Druggable Non-olfactory G certain ergot derivatives used in treating Parkinson disease and migraine headaches (e.g. pergolide, cabergoline, and dihydroer- Protein-coupled Receptor gotamine), we correctly predicted that these medications * would also induce valvular heart disease (7, 8). Two of these Superfamily drugs (pergolide and cabergoline) were withdrawn from the Published, JBC Papers in Press, June 22, 2015, DOI 10.1074/jbc.R115.654764 Bryan L. Roth1 and Wesley K. Kroeze international market following large-scale trials demonstrating From the Department of Pharmacology, University of North Carolina their life-threating side effects (8, 9). In follow-up studies, we Chapel Hill School of Medicine, Chapel Hill, North Carolina 27514 surveyed 2200 FDA-approved and investigational medications, finding that 27 had potentially significant 5-HT2B agonism, of G-protein-coupled receptors (GPCRs) are frequent and fruit- which 6 are currently FDA-approved (guanfacine, quinidine, ful targets for drug discovery and development, as well as being xylometazoline, oxymetazoline, fenoldopam, and ropinirole) off-targets for the side effects of a variety of medications. Much (10). Interestingly, of the 2200 drugs screened, around 30% dis- of the druggable non-olfactory human GPCR-ome remains played significant 5-HT2B antagonist activity (10), indicating under-interrogated, and we present here various approaches that 5-HT2B receptors represent a “promiscuous target” for that we and others have used to shine light into these previously approved and candidate medications. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented.