Early Cretaceous Lineages of Monocot Flowering Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Partial Flora Survey Rottnest Island Golf Course
PARTIAL FLORA SURVEY ROTTNEST ISLAND GOLF COURSE Prepared by Marion Timms Commencing 1 st Fairway travelling to 2 nd – 11 th left hand side Family Botanical Name Common Name Mimosaceae Acacia rostellifera Summer scented wattle Dasypogonaceae Acanthocarpus preissii Prickle lily Apocynaceae Alyxia Buxifolia Dysentry bush Casuarinacea Casuarina obesa Swamp sheoak Cupressaceae Callitris preissii Rottnest Is. Pine Chenopodiaceae Halosarcia indica supsp. Bidens Chenopodiaceae Sarcocornia blackiana Samphire Chenopodiaceae Threlkeldia diffusa Coast bonefruit Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Suada australis Seablite Chenopodiaceae Atriplex isatidea Coast saltbush Poaceae Sporabolis virginicus Marine couch Myrtaceae Melaleuca lanceolata Rottnest Is. Teatree Pittosporaceae Pittosporum phylliraeoides Weeping pittosporum Poaceae Stipa flavescens Tussock grass 2nd – 11 th Fairway Family Botanical Name Common Name Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Atriplex isatidea Coast saltbush Cyperaceae Gahnia trifida Coast sword sedge Pittosporaceae Pittosporum phyliraeoides Weeping pittosporum Myrtaceae Melaleuca lanceolata Rottnest Is. Teatree Chenopodiaceae Sarcocornia blackiana Samphire Central drainage wetland commencing at Vietnam sign Family Botanical Name Common Name Chenopodiaceae Halosarcia halecnomoides Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Sarcocornia blackiana Samphire Poaceae Sporobolis virginicus Cyperaceae Gahnia Trifida Coast sword sedge -

Restoration After Removal of Pines at Gnangara Final
RESTORATION OF BANKSIA WOODLAND AFTER THE REMOVAL OF PINES AT GNANGARA: SEED SPECIES REQUIREMENTS AND PRESCRIPTIONS FOR RESTORATION A report prepared on behalf of the Department of Environment and Conservation for the Gnangara Sustainability Strategy Kellie Maher University of Western Australia May 2009 Restoration of Banksia woodland after the removal of pines at Gnangara: seed species requirements and prescriptions for restoration Report for the Department of Environment and Conservation Kellie Maher University of Western Australia Gnangara Sustainability Strategy Taskforce Department of Water 168 St Georges Terrace Perth Western Australia 6000 Telephone +61 8 6364 7600 Facsimile +61 8 6364 7601 www.gnangara.water.wa.gov.au © Government of Western Australia 2009 May 2009 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any use as permitted under the Copyright Act 1968 , all other rights are reserved. Requests and inquiries concerning reproduction and rights should be addressed to the Department of Conservation and Environment. This document has been commissioned/produced as part of the Gnangara Sustainability Strategy (GSS). The GSS is a State Government initiative which aims to provide a framework for a whole of government approach to address land use and water planning issues associated with the Gnangara groundwater system. For more information go to www.gnangara.water.wa.gov.au 1 Restoration of Banksia woodland after the removal of pines at Gnangara: seed species requirements and prescriptions for restoration A report to the Department of Environment and Conservation Kellie Maher University of Western Australia May 2009 2 Table of Contents List of Tables .................................................................................................................... -

Plant Descriptions 2018 4/22/2018
Tyler Plant Sale - Plant Descriptions 2018 4/22/2018 TypeDesc Botanical Common Season of Exposure Size Description Name Name Interest Woody: Vine Clematis Clematis Summer to Sun to 8-10' Clematis 'Cardinal Wyszynski' dazzles your garden with huge 8" glowing 'Cardinal Fall Partial crimson flowers. The vibrant flowers are accented with darker crimson Wyszynski' Shade anthers and light pink filaments. Blooms in June-July and again in September. Attracts pollinators. Easy to grow in a rich, porous, alkaline soil. Provide shade for the roots with a generous layer of mulch or a shallow-rooted groundcover near the base of the vine. Received the Golden Medal at 'Plantarium' in 1990. Woody: Vine Clematis Hybrid Summer Sun to 6-8’ Fully double white flowers have yellow anthers and green outer petals. 'Duchess of Clematis Partial They are borne on the previous year’s growth and the current season’s Edinburgh' Shade new growth. This clematis does not require heavy pruning, remove only weak or dead stems in late spring. Tolerates most garden soils, needs protection from cold winds. Woody: Vine Clematis Clematis Early Sun to 8-10’ A beautiful, compact vine that covers itself with 5” shell pink flowers in 'Hagley Summer Partial summer. 'Hagley Hybrid' is also know as Pink Chiffon. This is a large- Hybrid' Shade flowering clematis that can be grown as a container plant. It is best keep out of full sun to prevent bleaching of flowers. Prefers moist, well-drained soil and for best results, mulch. TypeDesc Botanical Common Season of Exposure Size Description Name Name Interest Woody: Vine Clematis x Clematis Summer to Sun to 6-10' This deciduous hybrid clematis, has unusual and very striking deep blue durandii Fall Partial flowers with creamy stamens on a non-clinging, scrambling vine. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Flavonoid, Phenylethanoid and Iridoid Glycosides from Globularia Aphyllanthes
Flavonoid, Phenylethanoid and Iridoid Glycosides from Globularia aphyllanthes Hasan Kırmızıbekmeza, Carla Bassarellob, Sonia Piacenteb, Galip Akaydınc, and Ihsan˙ C¸ alıs¸d,e a Yeditepe University, Faculty of Pharmacy, Department of Pharmacognosy, TR-34755, Kayıs¸da˘gı, Istanbul,˙ Turkey b Salerno University, Department of Pharmaceutical Sciences, Via Ponte Don Melillo, 84084 Fisciano, Salerno, Italy c Hacettepe University, Department of Biology Education, TR-06800, Beytepe, Ankara, Turkey d Hacettepe University, Faculty of Pharmacy, Department of Pharmacognosy, TR-06100, Ankara, Turkey e Present address: Near East University, Faculty of Pharmacy, Department of Pharmacognosy and Pharmaceutical Botany, Nicosia, Turkish Republic of Northern Cyprus Reprint requests to Dr. Hasan Kırmızıbekmez. Fax: +90 216 578 00 68. E-mail: [email protected] Z. Naturforsch. 2009, 64b, 252 – 256; received September 4, 2008 A new flavone glycoside, 6-hydroxyluteolin 7-O-[6 -benzoyl-β-D-glucopyranosyl-(1 → 2)]-β- D-glucopyranoside (aphyllanthoside, 1) was isolated from the MeOH extract of the aerial parts of Globularia aphyllanthes. Besides this new compound, two flavonoid glycosides (6-hydroxyluteolin 7-O-[6 -(E)-caffeoyl-β-D-glucopyranosyl-(1 → 2)]-β-D-glucopyranoside and isoquercitrin), three phenylethanoid glycosides (verbascoside, rossicaside A, and trichosanthoside A), and 11 iridoid gly- cosides (aucubin, catalpol, 10-O-benzoylcatalpol, globularin, asperuloside, besperuloside, asperulo- sidic acid, daphylloside, scandoside, alpinoside and baldaccioside) were also obtained and character- ized. Identification of the isolated compounds was carried out by spectroscopic analysis including 1D and 2D NMR experiments as well as HRMS. Key words: Globularia aphyllanthes, Plantaginaceae, Flavonoids, Aphyllanthoside, Phenylethanoid and Iridoid Glycosides Introduction Experimental Section Globularia aphyllanthes Crantz (Plantaginaceae) is General experimental procedures a perennial plant, distributed from northwest Turkey to central and southern Europe [1]. -

Phd Federica Gilardelli A5
Vegetation dynamics and restoration trials in limestone quarries: the Botticino case study (Brescia, Italy) Federica Gilardelli UNIVERSITÀ DEGLI STUDI DI MILANO – BICOCCA Facoltà di Scienze Matematiche, Fisiche e Naturali Vegetation dynamics and restoration trials in limestone quarries: the Botticino case study (Brescia, Italy) Federica Gilardelli PhD thesis in Environmental Science XXV cycle Tutor: Cotutors: Prof. Sandra Citterio Prof. Sergio Sgorbati Dr. Rodolfo Gentili Dr. Stefano Armiraglio Collaborations: Dr. Ing. Sergio Savoldi Dr. Pierangelo Barossi February 2013 To all the quarrymen and their families. A tutti i cavatori e le loro famiglie. Background. All over the world, the naturalistic restoration of abandoned quarry areas represents a real challenge because of the very adverse initial site conditions for plant species colonization. In order to identify the best restoration practices, the present thesis considered, as a case study, the “Botticino extractive basin” (Lombardy, Italy), that is today the second greatest Italian extractive basin and it is famous worldwide for the limestone extraction. In particular, the thesis proposes a multidisciplinary approach based on the study of the local vegetation dynamics, laboratory tests, plant selection for restoration and field experiments to test different restoration techniques. Methods. Spontaneous vegetation dynamics over the whole extractive basin was studied by an ecological approach through 108 plots, that were carried out on surfaces whose “disused time” from quarry abandonment was known; data were analysed by cluster analysis and Canonical Correspondence Analysis (CCA) and compared to the available data on grassland and woodlands related to the study area. We identified successional phases according to the trend of the most common species whose cover significantly increases or decreases with time. -

1 Contrasting Habitat and Landscape Effects on the Fitness of a Long-Lived Grassland Plant Under 1 Forest Encroachment
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Diposit Digital de Documents de la UAB 1 Contrasting habitat and landscape effects on the fitness of a long-lived grassland plant under 2 forest encroachment: do they provide evidence for extinction debt? 3 4 Guillem Bagaria1,2*, Ferran Rodà1,2, Maria Clotet1, Silvia Míguez1 and Joan Pino1,2 5 6 1CREAF, Cerdanyola del Vallès 08193, Spain; 2Univ Autònoma Barcelona, Cerdanyola del Vallès 7 08193, Spain. 8 9 *Correspondence author. CREAF, Cerdanyola del Vallès 08193, Spain. 10 E-mail: [email protected] 11 Phone: +34 935814851 12 FAX: +34 93 5814151 13 14 Running title: Plant fitness and extinction debt 15 This is the accepted version of the following article: Bagaria, G. , et al.“Contrasting habitat and landscape effects on the fitness of a long-lived grassland plant under forest encroachment: do they provide evidence for extinction debt?” in Journal of ecology (Ed. Wiley), vol. 106, issue 1 (Jan. 2018), p. 278-288, which has been published in final form at DOI 10.1111/1365-2745.12860. 1 16 Summary 17 1. Habitat loss, fragmentation and transformation threaten the persistence of many species 18 worldwide. Population and individual fitness are often compromised in small, degraded and isolated 19 habitats, but extinction can be a slow process and extinction debts are common. 20 2. Long-lived species are prone to persist as remnant populations in low quality habitats for a long 21 time, but the population and individual-level mechanisms of extinction debt remain poorly explored 22 so far. -
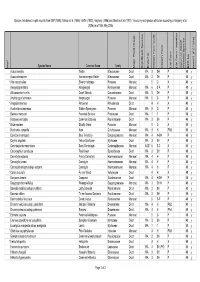
BFS048 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(S): Grass Phylogeny Working Group, Nigel P
Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(s): Grass Phylogeny Working Group, Nigel P. Barker, Lynn G. Clark, Jerrold I. Davis, Melvin R. Duvall, Gerald F. Guala, Catherine Hsiao, Elizabeth A. Kellogg, H. Peter Linder Source: Annals of the Missouri Botanical Garden, Vol. 88, No. 3 (Summer, 2001), pp. 373-457 Published by: Missouri Botanical Garden Press Stable URL: http://www.jstor.org/stable/3298585 Accessed: 06/10/2008 11:05 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=mobot. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected]. -

Download the Full Report Pdf, 2.9 MB
VKM Report 2016:50 Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Opinion of the Panel on Alien Organisms and Trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food Safety Report from the Norwegian Scientific Committee for Food Safety (VKM) 2016:50 Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Opinion of the Panel on Alien Organisms and Trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food Safety 01.11.2016 ISBN: 00000-00000 Norwegian Scientific Committee for Food Safety (VKM) Po 4404 Nydalen N – 0403 Oslo Norway Phone: +47 21 62 28 00 Email: [email protected] www.vkm.no www.english.vkm.no Suggested citation: VKM (2016). Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants. Scientific Opinion on the on Alien Organisms and Trade in Endangered species of the Norwegian Scientific Committee for Food Safety ISBN: 978-82-8259-240-6, Oslo, Norway. VKM Report 2016:50 Title: Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Authors preparing the draft opinion Hugo de Boer (chair), Maria G. Asmyhr (VKM staff), Hanne H. Grundt, Inga Kjersti Sjøtun, Hans K. Stenøien, Iris Stiers. Assessed and approved The opinion has been assessed and approved by Panel on Alien organisms and Trade in Endangered Species (CITES). Members of the panel are: Vigdis Vandvik (chair), Hugo de Boer, Jan Ove Gjershaug, Kjetil Hindar, Lawrence Kirkendall, Nina Elisabeth Nagy, Anders Nielsen, Eli K. -

Dyuhei Sato Division of Genetics, Bot. Inst. Faculty of Science, Tokyo
ANALYSIS OF THE KARYOTYPES IN YUCCA, A GA VE AND THE RELATED GENERA WITH SPECIAL REFERENCE TO THE PHYLOGENETIC SIGNIFICANCEI~ Dyuhei SATo Divisionof Genetics, Bot. Inst. Faculty of Science, Tokyo Imperial University McKelvey and Sax (2933) have called attention to the existence of taxonomic and cytological similarities of the genera Yucca, Hesperoyucca, Gleistvucca,Hesperoaloe and Samuela of the Liliaceae with the genera Agave and Fourcroya which belong to a related family, Amaryllidaceae. Wh.itaker (1934) also has reported that Polianhes and Fourcroya have exactly the same chromosome constitution as the Yucca-Abave karyotype (5 long and 25 short chromosomes) (Figs. 1, 2). These observations when considered in respect to taxonomic resemblances, seem to indicate that the genera mentioned above are more closely related than it is shown by their classifica- tion into distinct families. Whitaker also has remarked that Dasylirion (2n=38) and ATolina(2n=36) in Yucceae and Doryanthes (2n=36) in Agavoideae are of different karyotypes from the Yucca-Agave type. In the present work an analysis of the karyotypes in Liliaceous plants has been attempted and several karyotypes have been found in Scilloideae. Eucornis and Carassia have been selected with the purpose of discovering a possible connecting link between these genera and the Yucca-Agave group. In the present paper an analysis of the karyotypes of the following species is given. LILIACEAE Scilloideae 211 Fig. Euconis undulata 60=8L+8M+44S (4b)2) 3 Euconsispallidi ora 60=8L+8M+44S (4b) 4 Eucomispunctata 60=8L±8M+44S (4b) 5 Camassiaescrema 30=6L+24S (2b) 6 Yucceae Yuccafilamentosa 30 60=1OL+50S (2b) 1, 7 Yuccarecurvifolia 30 60=1OL+50S (2b) 2, 8 Yuccaaloifolia 60=1OL+50S (2b) 9 „ var. -

Winter 2004.Pmd
The Lady-Slipper, 19:4 / Winter 2004 1 The Lady-Slipper Kentucky Native Plant Society Number 19:4 Winter 2004 A Message from the President: It’s Membership Winter is upon us. I hope everyone had some opportunity to experience the colors of Fall and now some of us will turn our attention to winter botany. While I was unable to Renewal Time! attend, I understand that our Fall meeting at Shakertown Kentucky Native Plant Society with Dr. Bill Bryant from Thomas More College as the guest EMBERSHIP ORM speaker was a great success. M F Our Native Plant Certification program was relatively successful this Fall. Plant taxonomy failed to meet Name(s) ____________________________________ because the NKU’s Community Education Bulletin was Address ____________________________________ mailed too late for anyone to sign up for the course. The woody plants course did, however, have a successful run. City, State, Zip ______________________________ This coming Spring, we will be offering Basic Plant Taxonomy, Plant Communities and Spring Wildflowers of KY County __________________________________ Central Kentucky. Tel.: (home) ______________________________ You will see in this issue that we are promoting “Chinquapin” the newsletter of the Southern Appalachian (work) ______________________________ Botanical Society (SABS). SABS is an organization E-mail _______________________________ largely made up of professional botanists and produces a quarterly scholarly journal. The newsletter “Chinquapin” o Add me to the e-mail list for time-critical native plant news has more of a general interest approach much like our o Include my contact info in any future KNPS Member Directory newsletter but on a regional scale. In this issue we have Membership Categories: provided subscription information on page 7.